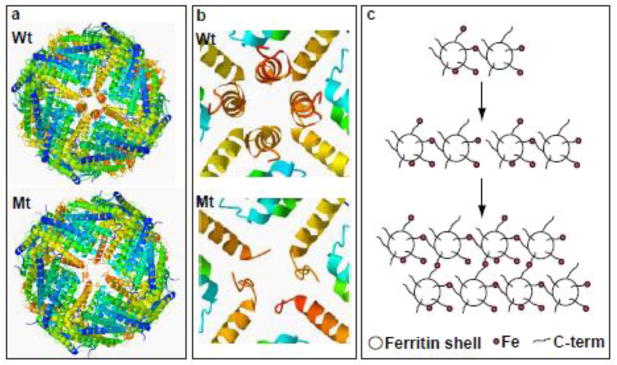
Figure 1. Ferritin structural disruption and aggregation caused by Mt-FTL subunits.
(a) The structure of the spherical shell is maintained in mutant ferritin as seen in thecrystallographic structures of Wt- and Mt-FTL homopolymers viewed down one of their 4-fold axes. (b) Close up views of the 4-fold pore from the interior of the Wt- and Mt-FTL structures show remarkable disruption in Mt-FTL making the pores unstable and leaky. Note that since the last 26 amino acids of Mt-FTL remained unaccounted for crystallographically, mutant C-termini are substantially longer than represented in (b), and if extended could reach as far as the diameter of the ferritin shell. (c) Iron loading-induced aggregation of Mt-FTL homopolymers is consistent with a model in which iron binds to the unraveled and extended portion of the mutant C-termini on two different ferritin shells bridging them and initiating a gradual accumulation of ferritin and iron into a precipitate. Bridging is not necessarily restricted to C-termini and may become more general, e.g., between a C-terminal group and a surface amino acid which both have affinity for iron. Structures were taken from RCSB (code 2FG8 for Wt-FTL and 3HX2 for Mt-FTL) and the precipitation model was modified from [14].