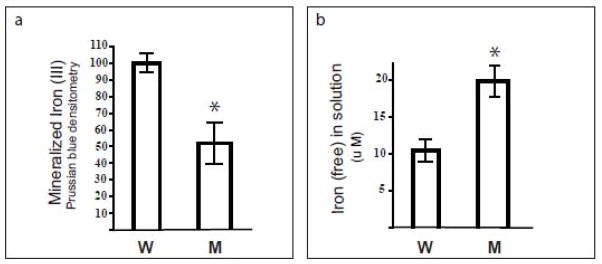
Figure 4. Iron incorporation by soluble ferritin heteropolymers.
Mt-FTL/FTH1 (M) and Wt-FTL/FTH1 (W) apoferritin heteropolymers were separately incubated with ferrous ammonium sulphate at concentrations of 1 μM and 1 mM, respectively. After 2 h, incubation was stopped by the addition of HCl and bathophenanthroline. (a) After separating unincorporated iron from the protein by electrophoresis on non-denaturing gels (3–8% native PAGE), iron biomineral within the heteropolymer interior was quantitated as density of Prussian blue formed in the protein bands. (b) Iron incorporation by the Mt-FTL/FTH1 heteropolymers relative to Wt-FTL/FTH1 was determined through measurement of residual ferrous iron in solution. Bathophenantholine was added to the samples after the 2 h incubation with iron, the samples were diluted 10-fold with buffer, their absorbances were read at 535 nm, and the amount of chelated Fe(II) was calculated using an extinction coefficient of 22140 M−1cm −1.