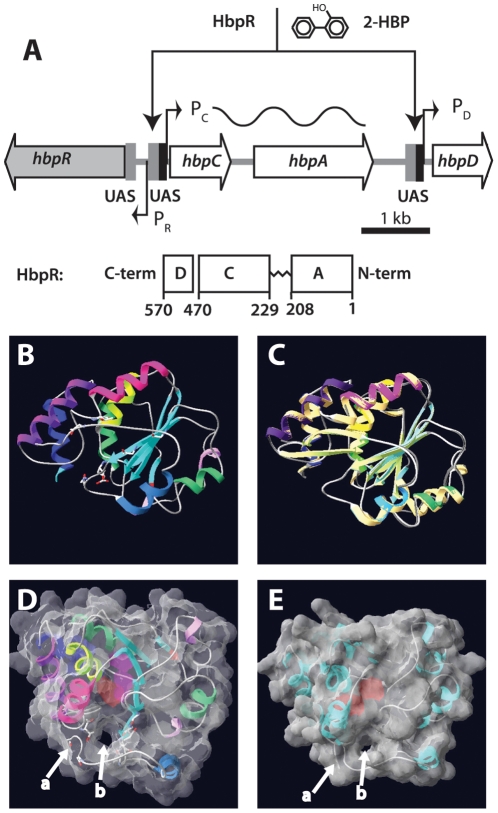
Figure 1. Overview of the hbp regulatory system and tertiary structure modeling of the HbpR A-domain using a previously established XylR model as template.
(A) Organization of the hbp genes and the location of the HbpR binding sites (UAS, upstream activating sequences) in front of the PC and PD promoters. HbpR domains are depicted to scale according to the predictions by Jaspers et al [7]. (B) to (E) Fitting used swiss-model and was performed on XylR A-domain PDB coordinates as calculated by Devos et al [22]. (B) Ribbon model of HbpR A-domain residues 11–209, with predicted coils, alpha-helices and beta-sheets indicated. (C) Superposition of the predicted HbpR and XylR A-domains in the same configuration as A. (D) Tertiary structure model of HbpR A-domain with calculated molecular surface at 1.4Å and 40% transparency, in order to see the helical, coil and sheets. Model turned into a position which enables visualization of the proposed tunnel entry (b). C-terminal end of coil ending the A-domain indicated with an arrow at (a). Pinkish region in the centre of the A-domain illustrates a predicted cavity within the A-domain. (E), as C but now for the XylR A-domain, with exception of the ten most C-terminal residues, which otherwise are predicted to occlude the tunnel.