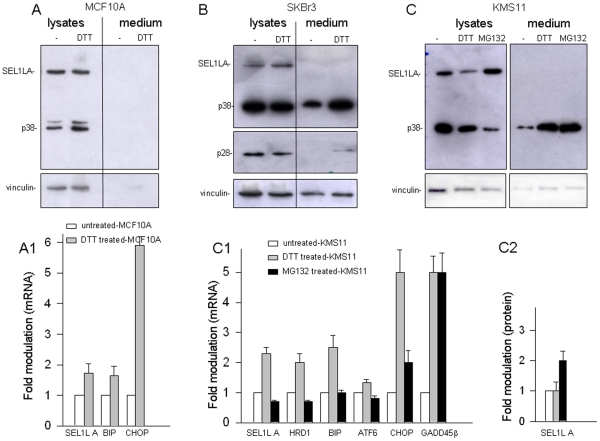
Figure 2. Analysis of p38 and p28 secretion in SKBr3, KMS11 and MCF10A cells exposed to chemical and pharmacological treatments.
A. Western blot analysis of untreated and DTT-treated MCF10A cells: MCF10A cells were exposed to DTT (2 mM) for 3 hours and successively maintained for 24 hrs in OPTIMEM. Secreted protein (50 µg) extracted from the culture medium by TCA precipitation, and aliquots of cell lysates (50 µg) were resolved by SDS-PAGE (10%) and blotted with monoclonal anti-SEL1L and anti-vinculin antibodies. P38 was not detectable in the culture medium, both in presence and in absence of DTT. In addition to p38, MCF10A cells showed a higher band, probably corresponding to an immature precursor or post-translationally-modified product. The image is representative of two independent experiments. A1. RT-PCR analysis of untreated and DTT-treated MCF10A cells: RNA was extracted from the samples described in panel A and analyzed by RT-PCR for the UPR response. The histogram shows expression values normalized relative to housekeeping signals and expressed as fold modulation relative to the untreated samples; densitometric analysis was performed by Scion imaging program. UPR activation upon DTT treatment is indicated by up-modulation of BIP and CHOP and XBP-1 splicing, concomitantly SEL1LA is incremented (gray bar). The corresponding images are shown in Figure S2A. The data are the averages of two different assays based on independent treatments, ±SD. B. Western blot analysis of untreated and DTT-treated SKBr3 cells: Secretion of p38 and p28 was evaluated in untreated and DTT-treated SKBr3 cells. Cells exposed to DTT (2 mM) for 3 hrs or not exposed were maintained for 24 hrs in OPTIMEM. Secreted protein (50 µg) extracted from the culture medium by TCA precipitation, and aliquots of cell lysates (50 µg) were resolved by SDS-PAGE (12%) and blotted with anti-SEL1L and anti-vinculin antibodies. ER stress/UPR strongly promoted secretion of p38 and, to a lesser extent, p28 in the culture medium. The image is representative of five independent experiments. C. Western blot analysis of KMS11 cells treated with DTT and MG132: KMS11 cells were exposed to DTT (2 mM) or MG132 (10 µM) for 3 hrs and successively maintained for 24 hrs in OPTIMEM. Secreted protein (30 µg), extracted from the culture medium by TCA precipitation, and aliquots of cell lysates (50 µg) were resolved by SDS-PAGE (10%) and blotted with anti-SEL1L and anti-vinculin antibodies. Both treatments markedly induced p38 secretion in the culture medium. The image is representative of five independent experiments. C1. RT-PCR analysis of KMS11 cells treated with DTT and MG132: RNA was extracted from the same samples described in panel C and analyzed by RT-PCR for the UPR response. The histogram shows expression values normalized relative to housekeeping signals and expressed as fold modulation relative to untreated samples; densitometric analysis was determined by Scion imaging program. UPR activation upon DTT treatment is indicated by the up-modulation of ATF6, BIP, and CHOP and by XBP-1 splicing (see Figure S2C), concomitantly the expression of SEL1LA, HRD1 and GADD45β is incremented (gray bar). The corresponding images are shown in Figure S2C. MG132 treatment did not trigger UPR activation, as indicated by the absence of ATF6 and BIP un-modulation and lack of XBP-1 splicing (see Figure S2C), nevertheless, CHOP and GADD45β were markedly up-modulated and SEL1LA and HRD1 down-modulated (black bars). The data are averages of four different assays based on independent treatments, ±SD. C2. SEL1LA protein expression in KMS11 cells treated with DTT and MG132: The histogram shows SEL1LA protein expression values obtained from the samples described in panel C, normalized relative to housekeeping signals and expressed as fold modulation relative to the untreated sample; densitometric analysis was performed using the Scion imaging program. MG132 determined SEL1LA protein accumulation up to 2 times relative to the control level (black bar). The data are the averages of four independent experiments, ±SD.