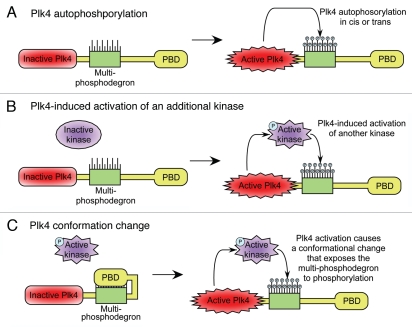
Figure 4.
Model for how Plk4 kinase activity autoregulates protein stability. (A) Kinase active Plk4 autophosphorylates the multi-phosphodegron. Phosphorylation promotes Plk4's ubiquitination by an E3 ligase and subsequent destruction by the proteasome. (B) Kinase active Plk4 phosphorylates and activates an additional kinase. This kinase subsequently phosphorylates the multi-phosphodegron leading to Plk4's downregulation. (C) Activation of Plk4 induces a conformational shift in the protein that exposes the previously masked multi-phosphodegron to phosphorylation by other kinases.