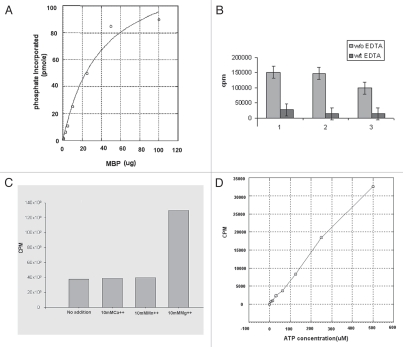
Figure 3.
Biochemical analysis of TcMAPK2. (A) Computer software analysis of enzymatic activity of TcMAPK2. Enzymatic parameters were calculated using KaleidaGraph 4 software. At the standard reaction conditions using 5 µg of the recombinant enzyme and varying concentration of MBP, the specific activity of recombinant TcMAPK2 is 6.4 ± 0.5 nmole PO4/incorp./µmole TcMAPK2/second and the apparent Km is in the nanomolar range (1.2 ± 0.2 nM). (B) EDTA abolished the enzymatic activity. Light color columns are reactions without EDTA while dark color columns with 250 mM EDTA. Three separate replicates were performed. (C) TcMAPK2 is Mg2+ dependent. 10 mM of either Ca2+ or Mn2+ shows no effect on the enzymatic activity as compared to standard kinase assay without adding divalent metal compounds while 10 mM Mg2+ significantly increases more than three folds of the enzymatic activity. The plot is a representative of three separate experiments. (D) TcMAPK2 is ATP dependent. Increase the concentration of ATP increases the incorporation of phosphate into the MBP. This linear curve is a representative of three separate experiments.