Table 3.
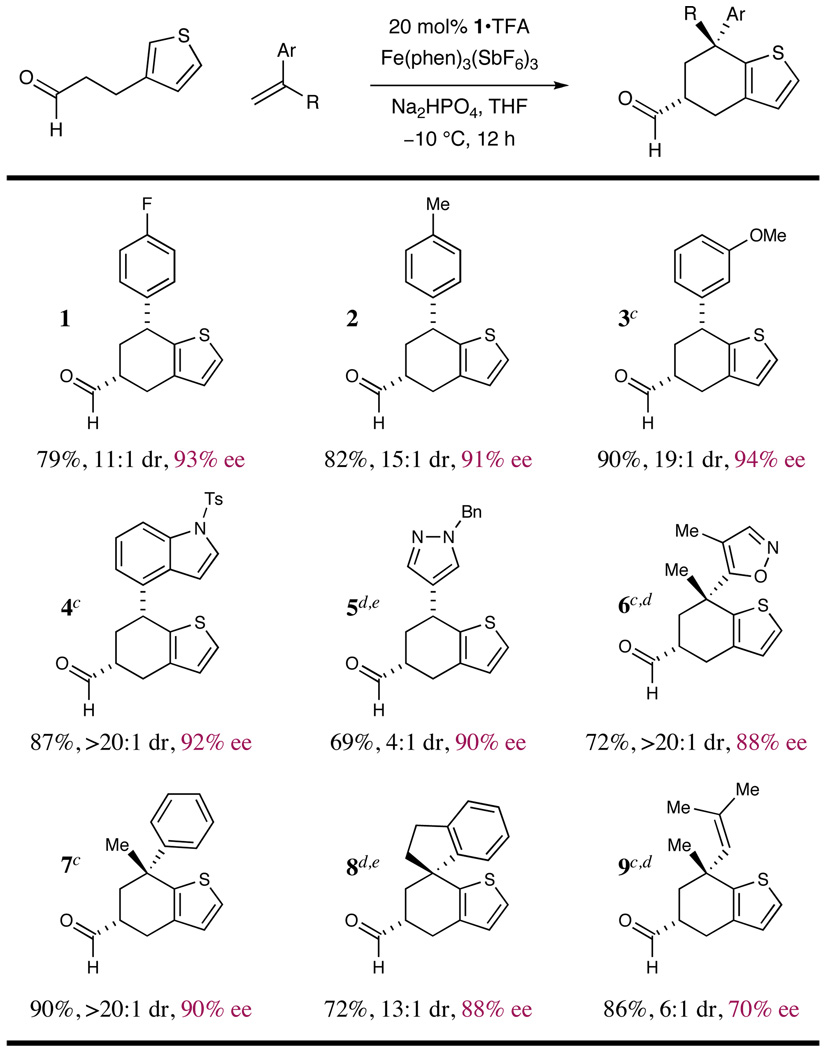 |
Results listed as product, yield, diastereomeric ratio (dr), enantiomeric excess (% ee).
Diastereomeric ratio, % ee determined as in Table 1.
Reaction conducted at −20 °C.
Reaction performed with Fe(phen)3(PF6)3 as oxidant.
Reaction conducted at −40 °C.
