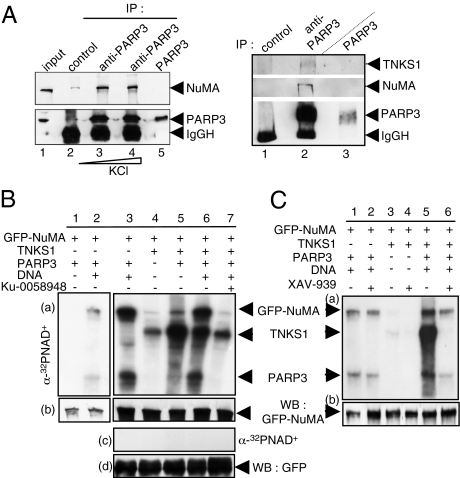
Fig. 2.
Physical and functional association of PARP3 with NuMA and tankyrase 1. (A Left) Coimmunoprecipitation of NuMA with PARP3 after increasing stringency conditions of washing buffers. Cos1 cell extracts were immunoprecipitated with a control antibody (lane 2) or an anti-PARP3 antibody (lanes 3 and 4) and analyzed by Western blotting using successively anti-NuMA and anti-PARP3 antibodies. Input corresponds to 1/13 of the total amount of cell extract used for immunoprecipitation. Lane 5, purified recombinant PARP3 (10 ng). (A Right) Coimmunoprecipitation of tankyrase 1 and NuMA with PARP3. Cos1 cell extracts were immunoprecipitated with a control antibody (lane 1) or an anti-PARP3 antibody (lane 2) and analyzed by Western blotting using successively anti-NuMA, anti-tankyrase 1 and anti-PARP3 antibodies. Lane 3, purified recombinant PARP3 (10 ng). (B) PARP3 induces the ADP ribosylation of NuMA both directly and through tankyrase 1. (a and b) Immunopurified GFP-NuMA was incubated with purified PARP3 and/or tankyrase 1 (TNKS1) as indicated in PARP activity buffer. The addition of Ku-0058948 (250 nM) significantly inhibits PARP3 but not tankyrase 1 (lane 7 vs. lanes 2 and 3). (c and d) In similar experimental conditions as above, no ADP ribosylation of GFP alone was detected. (a and c) Autoradiography. (b and d) Immunopurified GFP or GFP-NuMA were analyzed by Western blotting using an anti-GFP antibody. (C) PARP3 stimulates the auto-ADP ribosylation of tankyrase 1. Immunopurified GFP-NuMA were incubated with purified PARP3 or tankyrase 1 and assayed for PARP activity as above. The addition of XAV-939 (500 nM) inhibits efficiently tankyrase 1 but not PARP3 (compare lanes 4 with 3, and 2 with 1). (a) Autoradiography. (b) Immunopurified GFP-NuMA was analyzed by Western blotting using an anti-GFP antibody.