Abstract
BACKGROUND AND PURPOSE
Diaphragm muscle weakness occurs in patients with heart failure (HF) and is associated with exercise intolerance and increased mortality. Reduced sensitivity of diaphragm fibres to calcium contributes to diaphragm weakness in HF. Here we have investigated the ability of the calcium sensitizer levosimendan to restore the reduced calcium sensitivity of diaphragm fibres from rats with HF.
EXPERIMENTAL APPROACH
Coronary artery ligation in rats was used as an animal model for HF. Sham-operated rats served as controls. Fifteen weeks after induction of HF or sham operations animals were killed and muscle fibres were isolated from the diaphragm. Diaphragm fibres were skinned and activated with solutions containing incremental calcium concentrations and 10 µM levosimendan or vehicle (0.02% DMSO). Developed force was measured at each calcium concentration, and force–calcium concentration relationships were plotted.
KEY RESULTS
Calcium sensitivity of force generation was reduced in diaphragm muscle fibres from HF rats, compared with fibres from control rats (P < 0.01). Maximal force generation was ∼25% lower in HF diaphragm fibres than in control fibres (P < 0.05). Levosimendan significantly increased calcium sensitivity of force generation in diaphragm fibres from HF and control rats, without affecting maximal force generation.
CONCLUSIONS AND IMPLICATIONS
Levosimendan enhanced the force generating capacity of diaphragm fibres from HF rats by increasing the sensitivity of force generation to calcium concentration. These results provide strong support for testing the effect of calcium sensitizers on diaphragm muscle weakness in patients with HF.
Keywords: calcium sensitization, heart failure, respiratory muscle, skinned fibres
Introduction
Patients with heart failure (HF) commonly suffer from dyspnea, which significantly limits their daily life activities. Dyspnea is the sensation of breathlessness and results from an imbalance between the load on the respiratory muscles and their capacity to generate force (Moxham and Jolley, 2009). In patients with HF, both sides of the scales are oppositely affected. On the one hand, exercise increases the workload on the respiratory muscles in patients with HF to levels up to threefold higher than in normal subjects (Mancini et al., 1992). On the other hand, several clinical studies have demonstrated that inspiratory muscle strength is commonly reduced in patients with HF (Hughes et al. 1999; Carmo et al., 2001). Moreover, the reduction of force generating capacity strongly correlates with the severity of dyspnea (Mancini et al., 1992; McParland et al. 1992), exercise intolerance (Chua et al., 1995; Faggiano et al. 2001) and mortality risk (Meyer et al., 2001). Despite the clinical relevance of inspiratory muscle dysfunction, no effective treatment aiming at improving contractility of the inspiratory muscles is currently available.
The diaphragm is the most important inspiratory muscle. Recent work from our lab demonstrated that diaphragm contractility is impaired at the muscle fibre level in rats with HF (van Hees et al., 2007). Among other factors, the sensitivity of contractile proteins to calcium appeared to be reduced. As a result, force generation is reduced at a sub-maximal calcium level in diaphragm fibres from rats with HF. Because the diaphragm is sub-maximally loaded during normal breathing, reduced calcium sensitivity could contribute to impaired force generation of the diaphragm in vivo. Accordingly, improving calcium sensitivity of diaphragm muscle fibres could enhance contractile performance of the diaphragm in HF.
At present, levosimendan is the only calcium sensitizer approved for use in humans (∼40 countries worldwide). Levosimendan has been shown to improve cardiac contractility in vivo (Follath et al., 2002) and in vitro (Edes et al., 1995), by enhancing calcium sensitivity of force generation through binding with cardiac/slow troponin C (Sorsa et al., 2003). Very recently, we demonstrated that levosimendan improves contractility of human diaphragm muscle as well (van Hees et al., 2009), providing a rationale to test the effects of levosimendan on diaphragm contractility in HF. Our present results indeed demonstrate that levosimendan improved calcium sensitivity of force generation in diaphragm fibres from animals with HF.
Methods
HF animal model
All animal care and experimental procedures in this study were approved by the local Animal Ethics Committee, Radboud University, Nijmegen. Adult male Wistar rats (∼300 g) were anesthetized by inhalation of an isoflurane-oxygen mixture (2% to 5% isoflurane), orally intubated and mechanically ventilated. Ligation of the left coronary artery and sham operations were performed as described previously (van Hees et al., 2007).
Fifteen weeks after coronary ligation or sham operation, the rats were anesthetized with pentobarbital (70 mg·kg−1 IP), tracheally intubated and mechanically ventilated. Aortic pressure and left ventricular pressures were measured by a micromanometre-tipped catheter (SPC 330, Millar Instruments, Houston, TX, USA) inserted through the right carotid artery. After completion of the haemodynamic measurements, a combined thoracotomy/laparotomy was performed, and the diaphragm, lungs and heart were quickly excised. A muscle bundle was cut from the right hemi-diaphragm for determination of single fibre contractile properties. Heart weight was determined and the heart was fixed in formalin for >72 h. Subsequently, the size of the infarct areas was determined by planimetry, as described previously (Pfeffer et al., 1979). Rats with infarcts >35% of the left ventricle were included in the HF group, and sham-operated rats served as controls. Lungs were cleaned of fat and weighed before and after desiccation (3 days at 40°C) to quantify lung oedema.
Single fibre contractile measurements
Single fibre contractile measurements and experimental protocols were as described earlier (van Hees et al., 2007, 2009), with minor modifications, as described as follows.
Single fibre preparation
The muscle bundles were excised quickly and immediately immersed in oxygenated Krebs solution at pH ∼7.40, cooled on ice. The Krebs solution consisted of 137 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM KH2PO4, 24 mM NaHCO3, 7 mM glucose and 25 µM d-tubocurarine (Sigma, Bornem, Belgium). A bundle was dissected from the central part of the muscle. The muscle bundle was pinned to cork and stored at 4°C in a relaxing solution, containing 50% glycerol (v/v). After 24 h, the muscle strip was stored at −20°C for later analysis. Approximately one hour prior to determination of single fibre contractile properties, the muscle bundle was transferred to relaxing solution (5°C) containing 1% Triton X-100 to permeabilize lipid membranes. From the muscle bundle ∼2 mm segments of single fibres were isolated using microforceps. Subsequently, the fibre ends were attached to aluminium foil clips, and mounted on the single fibre apparatus. Fibres were mounted in a temperature-controlled (20°C) flow-through acrylic chamber (120 µL volume), with a glass coverslip bottom, on the stage of an inverted microscope (model IX-70; Olympus, Amsterdam, the Netherlands). Two stainless steel hooks were used to mount the fibre horizontally in the chamber. One end of the fibre was attached to a force transducer (model AE-801; SensoNor, Horten, Norway) with a resonance frequency of 10 kHz, whereas the other end was attached to a servomotor (model 308B; Aurora Scientific, Aurora, ON, Canada) with a step time of 250 µs. In relaxing solution, sarcomere length was set at 2.4 µm as the optimal length for force generation (Zuurbier et al. 1995; Burkholder and Lieber, 2001) with the use of a calibrated eyepiece micrometer. During experiments, sarcomere length was stabilized with the Brenner cycling method (Brenner, 1983) as modified by Sweeney et al. (1987). MIDAC software (Radboud University, Nijmegen, the Netherlands) and a data-acquisition board were used to record signals. Muscle fibre length (∼1–1.5 mm) was measured using a reticule in the microscope eyepiece [×10 Olympus Plan 10, 0.30 numerical aperture (NA)]. The XY fibre diameter (width) was measured with a ×40 objective (×40 Olympus Plan 40, 0.60 NA). The ×40 objective also was used to measure the XZ fibre diameter (depth) by noting the displacement of the microscope's objective while focusing on the top and bottom surfaces of the fibre. Three width and depth measurements were made along the length of the fibre, the average values were used to calculate the fibre cross-sectional area, assuming that the fibre was ellipsoid in shape.
Composition of solutions for single fibre measurements
Concentrated stock solutions of levosimendan (kind gift from Orion Pharma, Espoo, Finland) were prepared in DMSO and stored at −20°C. Before use, levosimendan stock solutions were diluted with experimental solutions. The response of 10 soleus fibres from a normal rat to different doses of levosimendan was determined during sub-maximal activation (at pCa 6.5). Figure 1 shows that levosimendan enhanced sub-maximal force generation with a maximal effect at 10 µM, which is similar to previously reported dose-response curves for human diaphragm fibres (van Hees et al., 2009) and cardiac fibres (Edes et al., 1995). Accordingly, further experiments were conducted with either 10 µM levosimendan or vehicle (0.02% DMSO). Relaxing solution consisted of 1.0 mM MgCl2, 4.0 mM Na2ATP, 5 mM EGTA, 10 mM imidazole, 15 mM creatine phosphate and sufficient KCL to adjust the total ionic strength to 150 mM at pH 7.0. The negative logarithm of the free Ca2+ concentration (pCa) of the relaxing solution was 9.0, while in activating solutions, the pCa ranged from 7.0 to 4.5 (with maximal activation at pCa 4.5). To achieve appropriate pCa in activating solutions, sufficient CaCl2 was added to solutions bathing the fibres.
Figure 1.
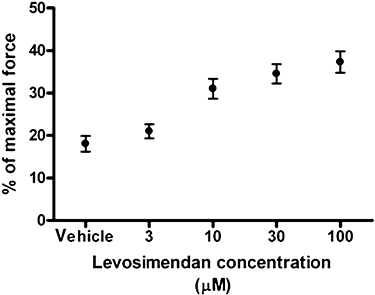
Dose-response effects of levosimendan on sub-maximal force generating capacity of rat soleus fibres. Incremental levosimendan concentrations were added to rat soleus fibres (n = 10) during sub-maximal activation at pCa 6.5. Force generation increases with rising levosimendan concentration, with a significant effect at 10 µM.
Diaphragm muscle single fibre experimental protocol
Maximal force was determined by perfusing the experimental chamber at, successively, pCa 9 and pCa 4.5. Then pCa 9.0 solution was perfused through the chamber to relax the fibre. Subsequently, fibres were perfused with solutions containing incremental Ca2+ concentrations. Each time a plateau was reached, the next pCa solution was perfused through the experimental chamber. Structural and functional instability of skinned single muscle fibres, reflected by an increased heterogeneity of sarcomere spacing during activation and reduced force generation over time, is a well-recognized problem (Brenner, 1998). To improve functional stability of muscle fibres, we applied Brenner cycling (Brenner, 1983) throughout the experimental protocol. Accordingly, no functional decline in maximal force generation over time was observed.
Diaphragm muscle single fibre contractile determinations
Maximum specific force was determined by dividing the isometric force generated at pCa 4.5 by cross-sectional area. The force-pCa relationship was derived by plotting recorded force against pCa of the corresponding activating solution. Graphpad software (Graphpad Software Inc., San Diego, CA, USA) was used to calculate the Ca2+ concentration required for half-maximum activation (pCa50), as an index of Ca2+ sensitivity of force generation and the Hill coefficient, as a measure of myofilament cooperativity.
Myosin heavy chain isoform composition determination
Because pCa50 is significantly different between slow and fast diaphragm fibres (Geiger et al., 1999). Diaphragm fibres were designated as slow or fast on the basis of myosin heavy chain isoform determination. Determination of myosin heavy chain isoform composition and content by SDS-PAGE was adapted from Geiger et al. (Geiger et al., 2000) and described previously (van Hees et al., 2007). From 46 control diaphragm fibres that were used in this study, 22 were designated as slow and 24 as fast. From 51 HF diaphragm fibres that were used, 37 were designated as slow and 14 as fast.
Data analysis
Differences regarding force-pCa relations between groups were statistically analysed with a two-way repeated measures (analysis of variance anova with pCa as the repeated measures factor and the presence of levosimendan as the second factor. One-way anova with post hoc Student-Newman-Keuls testing was performed to evaluate the statistical significance of differences between groups of other contractile characteristics. A probability level of P < 0.05 was considered significant. Mean ± SEM values are presented in text, tables and figures.
Results
Animal characteristics
Animal characteristics are shown in Table 1. HF was characterized by pulmonary congestion, increased heart weight, elevated left ventricular end diastolic pressure, decreased left ventricular peak systolic pressure and decreased aortic pressures.
Table 1.
Haemodynamic and physical characteristics of the two groups of rats
| Control (n = 5) | Heart failure (n = 5) | |
|---|---|---|
| Body weight (pre-op) (g) | 279 ± 7 | 282 ± 8 |
| Body weight (after 15 weeks) (g) | 443 ± 19 | 427 ± 9 |
| Aorta systolic pressure (mmHg) | 85 ± 5 | 67 ± 3* |
| Aorta diastolic pressure (mmHg) | 66 ± 5 | 52 ± 4* |
| LV peak systolic pressure (mmHg) | 98 ± 7 | 76 ± 4* |
| LV end diastolic pressure (mmHg) | 1 ± 2 | 9 ± 3* |
| Heart weight (g) | 1.4 ± 0.1 | 2.5 ± 0.2* |
| Infarct size (macroscopic) (%) | – | 41 ± 3 |
| Lung weight wet to dry ratio | 4.3 ± 0.1 | 4.9 ± 0.1* |
LV, Left ventricle.
P < 0.05, different from corresponding value in control group; Student's t-test.
Levosimendan and calcium sensitivity in HF diaphragm
Levosimendan increased calcium sensitivity of force generation in diaphragm fibres from HF and control animals, which is indicated by a leftward shift of the force-pCa relations in levosimendan exposed fibres (Figure 2). During sub-maximal activation, for instance at pCa 6.0, levosimendan increased diaphragm fibre force by 15–25% compared with vehicle-exposed fibres. Accordingly, the pCa50 was higher in levosimendan-treated fibres than in vehicle-treated fibres, for both HF and control fibres (Figure 3), which means that less calcium is needed to generate 50% of maximal force. Separation of the results into groups expressing slow or fast myosin heavy chain shows that the effect of levosimendan was similar among different fibre types.
Figure 2.
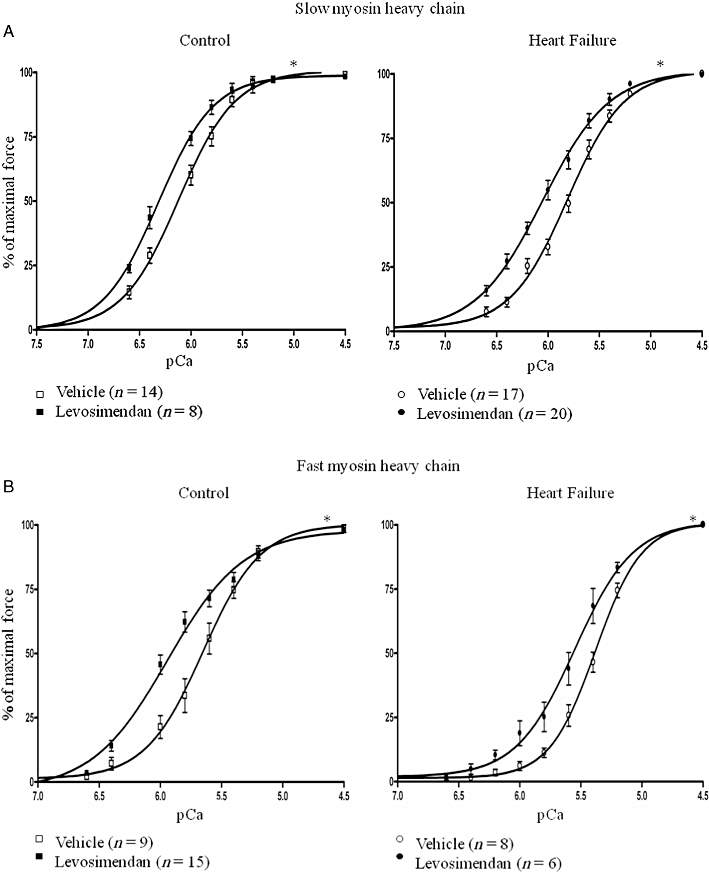
The effect of levosimendan on force-calcium characteristics of diaphragm muscle fibres from rats with heart failure and control rats. Diaphragm fibres were exposed to 10 µM levosimendan or vehicle activating solutions. Isometric force generation in response to solutions with incremental calcium concentrations was determined. The force-pCa relations of diaphragm fibres treated with levosimendan showed increased sensitivity to calcium force-pCa relations of diaphragm fibres treated with vehicle, for both slow (Figure 2A) and fast (Figure 2B) fibres. *P < 0.05, significant effect of levosimendan; two-way repeated measures anova.
Figure 3.
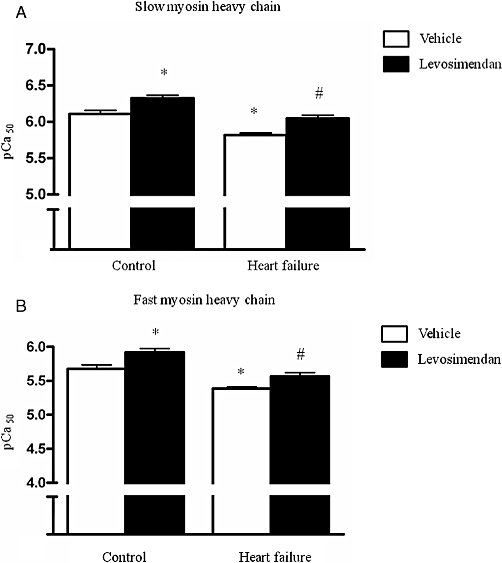
Values of pCa50, an index for calcium sensitivity of force generation, from control and HF diaphragm fibres exposed to either 10 µM levosimendan or vehicle solution. Calcium sensitivity was lower in HF fibres than in control fibres. Diaphragm fibres treated with levosimendan displayed higher calcium sensitivity than diaphragm fibres treated with vehicle, for both slow (Figure 3A) and fast (Figure 3B) fibres. *P < 0.05 compared with control vehicle-treated fibres; #P < 0.05 compared with vehicle treated HF fibres. pCa50 = −10log[Ca2+] at which 50% of maximal force is generated.
Myofilament cooperativity, reflected by the Hill coefficient was not significantly different between vehicle and levosimendan treated fibres; for slow control fibres (1.8 ± 0.1 vs. 2.1 ± 0.2), for slow HF fibres (1.8 ± 0.2 vs. 1.6 ± 0.1), for fast control fibres (2.1 ± 0.2 vs. 2.1 ± 0.6) and for fast HF fibres (2.4 ± 0.2 vs. 2.2 ± 0.3).
Also, maximal force generation was not affected by exposure to levosimendan in both HF and control fibres (Figure 4).
Figure 4.
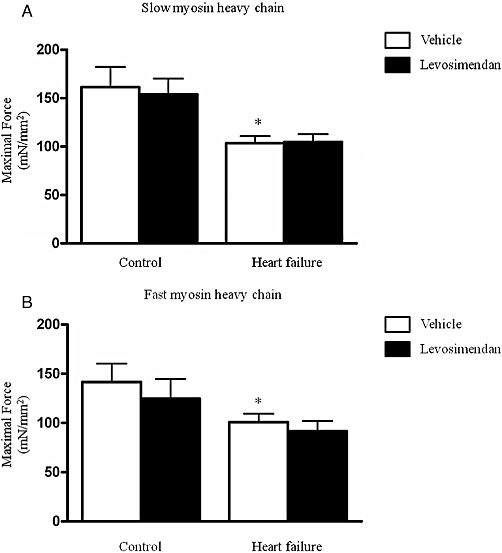
Maximal force generation of control diaphragm fibres and HF diaphragm fibres exposed to 10 µM levosimendan or vehicle solution. HF diaphragm fibres generated significantly lower maximal forces than control fibres, in both slow and fast fibres (Figure 4A and B, respectively). Maximal force generation was not significantly different between levosimendan and vehicle-treated HF fibres. *P < 0.05 compared with vehicle-treated control fibres.
HF versus control diaphragm
Levosimendan improved calcium sensitivity of HF and control diaphragm fibres to similar extent in both slow and fast typed fibres (Figure 3). Vehicle-treated fibres from HF rats displayed lower calcium sensitivity and maximal force generating capacity than vehicle treated fibres from control rats (Figures 3 and 4), which confirmed our earlier data (van Hees et al., 2007).
Discussion
This study is the first to show that the calcium sensitizer levosimendan improves calcium sensitivity of force generation in the diaphragm muscle of HF rats. Many studies have shown that levosimendan improves cardiac contractility, but very few studies have evaluated the effect of levosimendan on non-cardiac muscle. The data of the present study provide a strong support for the testing of the effects of calcium sensitizers on diaphragm muscle function in patients with congestive heart failure.
The workload on inspiratory muscles in HF patients is increased threefold compared with healthy subjects (Mancini et al., 1992). An increased workload demands higher oxygen consumption by inspiratory muscles (Collett et al., 1985), which, in turn, will reduce energy efficiency of ventilation. Indeed, energy efficiency of ventilation is significantly reduced in most patients with HF and significantly correlates with exercise limitation and mortality (Al-Rawas et al., 1995; Kurotobi et al., 1997; Wasserman et al., 1997; Kleber et al. 2000; Meyer et al. 2000). Therefore, it is relevant to seek for therapies that aim to improve efficiency of inspiratory muscle contractility. In vivo, the diaphragm muscle undergoes repetitive cycles of contraction and relaxation, which requires repetitive sequestration of calcium at the muscle fibre level. Calcium cycling is a highly energy consuming process, comprising 30–40% of energy cost during muscle contraction (Barclay et al., 2007). When calcium sensitivity is reduced, as it is in HF diaphragm fibres (van Hees et al., 2007), more calcium is required to generate force, which clearly reduces the mechanical efficiency of contraction. Conversely, by increasing the sensitivity of myofilaments to calcium, the same amount of force can be generated with less calcium, thereby improving the efficiency of muscle contraction. The present study demonstrates that levosimendan indeed increases the calcium sensitivity of diaphragm fibres in HF. Whether this increases efficiency of respiratory muscle work in HF patients remains to be investigated. It is promising that in cardiac muscle levosimendan increases cardiac output without increasing energy expenditure (Ukkonen et al. 1997; Ukkonen et al., 2000; Kaheinen et al., 2004).
Mechanisms of action
Levosimendan increases calcium sensitivity by interaction with the troponin complex, which regulates muscle contraction. The binding of levosimendan to troponin stabilizes the conformation of the troponin complex during cross-bridge formation (Sorsa et al., 2004). Previous studies have shown that levosimendan interacts with cardiac troponin C. This isoform of troponin C is also expressed in slow type skeletal muscle fibres, which would explain the positive effect of levosimendan on calcium sensitivity in slow type diaphragm fibres. However, fast type rat diaphragm fibres predominantly express the fast isoform of troponin C (Geiger et al., 1999) of which 35% of the amino acid composition is different from the slow isoform (Roher et al., 1986). Although the ability of levosimendan to interact with fast troponin C has never been investigated, the present results on the effect of levosimendan in fast type diaphragm fibres indicate that levosimendan interacts with fast troponin C as well. This finding has potential clinical importance, since the human diaphragm is composed of both slow and fast typed muscle fibres expressing slow and fast troponin C.
As expected, levosimendan did not affect maximal force generation, indicating that the maximal number of attached cross-bridges, and force per cross-bridge remain unaltered with levosimendan exposure. Accordingly, levosimendan improves the sub-maximal force generating capacity of skeletal muscle and HF diaphragm fibres by increasing sensitivity to calcium.
Study limitations
The effect of levosimendan on skeletal muscle contractility was studied in skinned muscle fibres, which brings several advantages and limitations. The most important feature of levosimendan is its capability to improve calcium sensitivity. The skinned muscle fibre preparation provides an excellent model to study calcium sensitivity, because force generation solely depends on myofilament function and the influence of other determinants, such as neuromuscular transmission and intracellular calcium signalling, are excluded. In addition to being a calcium sensitizer, levosimendan affects other cellular processes, including opening of ATP-sensitive potassium channels (Yokoshiki et al., 1997) which explains its vasodilatory actions in vivo (Michaels et al., 2005). Levosimendan is a also a selective inhibitor of phosphodiesterase-3. However, it was shown that phosphodiesterase-3 inhibition alone does not contribute to its inotropic effects on the heart muscle (Szilagyi et al., 2005). When muscle fibres are chemically skinned, as in our experimental preparation, membrane channels lose functionality and the intracellular milieu of the muscle fibre is replaced by activating solutions. Accordingly, the effects of levosimendan in the current study cannot be related to its effects on potassium channels and phosphodiesterases in diaphragm muscle. Considering these potential side effects of levosimendan, it is obvious that the extrapolation of the present in vitro data to effects on diaphragm contractility in vivo needs to be carried out with care. Nevertheless, the results from this study provide a rationale to further explore the feasibility of levosimendan treatment in patients with inspiratory muscle dysfunction.
Clinical applicability
Recently, we showed that levosimendan improves in vitro calcium sensitivity of human diaphragm fibres (van Hees et al., 2009). Levosimendan is generally well tolerated, with most reported side effects being hypotension and headache, but these have only been investigated in patients with HF (Nieminen et al., 2000; Follath et al., 2002; Moiseyev et al., 2002). Dose-response relations in human diaphragm fibres indicate that levosimendan improves force generation at concentrations above 0.1 µM (van Hees et al., 2009). Similar values of levosimendan (up to 0.4 µM) in plasma have been reported after administration of clinically used dosages (24 h infusion with 0.1–0.4 µg·kg−1·min−1) (Kivikko et al., 2003). However, intracellular levosimendan concentrations would seem to be more relevant than its blood levels. These are difficult to determine because in vivo levosimendan is rapidly metabolized to OR-1855, which is subsequently acetylated into OR-1896 (Antila et al., 2007). OR-1896 is known to have similar effects on calcium sensitization in cardiac muscle as levosimendan (Szilagyi et al., 2004), but has a much longer half-life (Antila et al., 2007). If OR-1896 also improves calcium sensitivity in skeletal muscle is yet unknown.
In addition to improving inspiratory muscle function, our data on concentration-response relations in soleus muscle fibres indicate that levosimendan also improves peripheral muscle function. In fact, calcium desensitization of peripheral muscles has been described in fatigued mice with HF (Lunde et al., 2006). Furthermore, levosimendan also improves contractility of diaphragm fibres from control animals. These data underline the rationale of testing the effect of levosimendan in other diseases associated with skeletal muscle dysfunction.
In conclusion, the present study demonstrated that levosimendan increased calcium sensitivity of diaphragm fibres in an animal model for human HF. These results imply that levosimendan would improve the energy efficiency of diaphragm force generation in vivo. Further investigations are needed to establish the applicability of levosimendan as a therapeutic agent in conditions associated with inspiratory muscle failure.
Acknowledgments
The authors of this manuscript wish to thank Dr J. Levijoki from Orion Pharma, Espoo, Finland for generously providing levosimendan and Leo Ennen from the Central Animal Lab, Radboud University Nijmegen, the Netherlands for performing the coronary artery ligations.
Glossary
Abbreviations
- DMSO
Dimethylsulfoxide
- HF
heart failure
- pCa
−10log[Ca2+]
Conflicts of interest
None
Supporting Information
Teaching Materials; Figs 1–4 as PowerPoint slide.
References
- Al-Rawas OA, Carter R, Richens D, Stevenson RD, Naik SK, Tweddel A, et al. Ventilatory and gas exchange abnormalities on exercise in chronic heart failure. Eur Respir J. 1995;8:2022–2028. doi: 10.1183/09031936.95.08122022. [DOI] [PubMed] [Google Scholar]
- Antila S, Sundberg S, Lehtonen LA. Clinical pharmacology of levosimendan. Clin Pharmacokinet. 2007;46:535–552. doi: 10.2165/00003088-200746070-00001. [DOI] [PubMed] [Google Scholar]
- Barclay CJ, Woledge RC, Curtin NA. Energy turnover for Ca2+ cycling in skeletal muscle. J Muscle Res Cell Motil. 2007;28:259–274. doi: 10.1007/s10974-007-9116-7. [DOI] [PubMed] [Google Scholar]
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983;41:99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Muscle mechanics II: skinned muscle fibers. In: Sugi H, editor. Current Methods In Muscle Physiology. Oxford: Oxford University Press; 1998. pp. 33–69. [Google Scholar]
- Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol. 2001;204:1529–1536. doi: 10.1242/jeb.204.9.1529. [DOI] [PubMed] [Google Scholar]
- Carmo MM, Barbara C, Ferreira T, Branco J, Ferreira S, Rendas AB. Diaphragmatic function in patients with chronic left ventricular failure. Pathophysiology. 2001;8:55–60. doi: 10.1016/s0928-4680(01)00065-7. [DOI] [PubMed] [Google Scholar]
- Chua TP, Anker SD, Harrington D, Coats AJ. Inspiratory muscle strength is a determinant of maximum oxygen consumption in chronic heart failure. Br Heart J. 1995;74:381–385. doi: 10.1136/hrt.74.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett PW, Perry C, Engel LA. Pressure-time product, flow, and oxygen cost of resistive breathing in humans. J Appl Physiol. 1985;58:1263–1272. doi: 10.1152/jappl.1985.58.4.1263. [DOI] [PubMed] [Google Scholar]
- Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, et al. Effects of Levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res. 1995;77:107–113. doi: 10.1161/01.res.77.1.107. [DOI] [PubMed] [Google Scholar]
- Faggiano P, d'Aloia A, Gualeni A, Giordano A. Relative contribution of resting haemodynamic profile and lung function to exercise tolerance in male patients with chronic heart failure. Heart. 2001;85:179–184. doi: 10.1136/heart.85.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/s0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol. 1999;87:1894–1900. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- van Hees HW, van der Heijden HF, Ottenheijm CA, Heunks LM, Pigmans CJ, Verheugt FW, et al. Diaphragm single-fiber weakness and loss of myosin in congestive heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H819–H828. doi: 10.1152/ajpheart.00085.2007. [DOI] [PubMed] [Google Scholar]
- van Hees HW, Dekhuijzen PN, Heunks LM. Levosimendan enhances force generation of diaphragm muscle from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:41–47. doi: 10.1164/rccm.200805-732OC. [DOI] [PubMed] [Google Scholar]
- Hughes PD, Polkey MI, Harrus ML, Coats AJ, Moxham J, Green M. Diaphragm strength in chronic heart failure. Am J Respir Crit Care Med. 1999;160:529–534. doi: 10.1164/ajrccm.160.2.9810081. [DOI] [PubMed] [Google Scholar]
- Kaheinen P, Pollesello P, Levijoki J, Haikala H. Effects of levosimendan and milrinone on oxygen consumption in isolated guinea-pig heart. J Cardiovasc Pharmacol. 2004;43:555–561. doi: 10.1097/00005344-200404000-00011. [DOI] [PubMed] [Google Scholar]
- Kivikko M, Lehtonen L, Colucci WS. Sustained hemodynamic effects of intravenous levosimendan. Circulation. 2003;107:81–86. doi: 10.1161/01.cir.0000043245.00859.11. [DOI] [PubMed] [Google Scholar]
- Kleber FX, Vietzke G, Wernecke KD, Bauer U, Opitz C, Wensel R, et al. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation. 2000;101:2803–2809. doi: 10.1161/01.cir.101.24.2803. [DOI] [PubMed] [Google Scholar]
- Kurotobi T, Sato H, Yokoyama H, Li D, Koretsune Y, Ohnishi Y, et al. Respiratory oxygen cost for dead space challenge is characteristically increased during exercise in patients with chronic heart failure: does it further decrease exercise capacity? J Card Fail. 1997;3:181–188. doi: 10.1016/s1071-9164(97)90014-2. [DOI] [PubMed] [Google Scholar]
- Lunde PK, Sejersted OM, Thorud HM, Tonnessen T, Henriksen UL, Christensen G, et al. Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue. Circ Res. 2006;98:1514–1519. doi: 10.1161/01.RES.0000226529.66545.e5. [DOI] [PubMed] [Google Scholar]
- McParland C, Krishnan B, Wang Y, Gallagher CG. Inspiratory muscle weakness and dyspnea in chronic heart failure. Am Rev Respir Dis. 1992;146:467–472. doi: 10.1164/ajrccm/146.2.467. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Henson D, LaManca J, Levine S. Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation. 1992;86:909–918. doi: 10.1161/01.cir.86.3.909. [DOI] [PubMed] [Google Scholar]
- Meyer FJ, Zugck C, Haass M, Otterspoor L, Strasser RH, Kubler W, et al. Inefficient ventilation and reduced respiratory muscle capacity in congestive heart failure. Basic Res Cardiol. 2000;95:333–342. doi: 10.1007/s003950070053. [DOI] [PubMed] [Google Scholar]
- Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kubler W, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation. 2001;103:2153–2158. doi: 10.1161/01.cir.103.17.2153. [DOI] [PubMed] [Google Scholar]
- Michaels AD, McKeown B, Kostal M, Vakharia KT, Jordan MV, Gerber IL, et al. Effects of intravenous levosimendan on human coronary vasomotor regulation, left ventricular wall stress, and myocardial oxygen uptake. Circulation. 2005;111:1504–1509. doi: 10.1161/01.CIR.0000159252.82444.22. [DOI] [PubMed] [Google Scholar]
- Moiseyev VS, Poder P, Andrejevs N, Ruda MY, Golikov AP, Lazebnik LB, et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN) Eur Heart J. 2002;23:1422–1432. doi: 10.1053/euhj.2001.3158. [DOI] [PubMed] [Google Scholar]
- Moxham J, Jolley C. Breathlessness, fatigue and the respiratory muscles. Clin Med. 2009;9:448–452. doi: 10.7861/clinmedicine.9-5-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1903–1912. doi: 10.1016/s0735-1097(00)00961-x. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, et al. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- Roher A, Lieska N, Spitz W. The amino acid sequence of human cardiac troponin-C. Muscle Nerve. 1986;9:73–77. doi: 10.1002/mus.880090112. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Pollesello P, Permi P, Drakenberg T, Kilpelainen I. Interaction of levosimendan with cardiac troponin C in the presence of cardiac troponin I peptides. J Mol Cell Cardiol. 2003;35:1055–1061. doi: 10.1016/s0022-2828(03)00178-0. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Pollesello P, Solaro RJ. The contractile apparatus as a target for drugs against heart failure: interaction of levosimendan, a calcium sensitiser, with cardiac troponin c. Mol Cell Biochem. 2004;266:87–107. doi: 10.1023/b:mcbi.0000049141.37823.19. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Corteselli SA, Kushmerick MJ. Measurements on permeabilized skeletal muscle fibers during continuous activation. Am J Physiol. 1987;252:C575–C580. doi: 10.1152/ajpcell.1987.252.5.C575. [DOI] [PubMed] [Google Scholar]
- Szilagyi S, Pollesello P, Levijoki J, Kaheinen P, Haikala H, Edes I, et al. The effects of levosimendan and OR-1896 on isolated hearts, myocyte-sized preparations and phosphodiesterase enzymes of the guinea pig. Eur J Pharmacol. 2004;486:67–74. doi: 10.1016/j.ejphar.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Szilagyi S, Pollesello P, Levijoki J, Haikala H, Bak I, Tosaki A, et al. Two inotropes with different mechanisms of action: contractile, PDE-inhibitory and direct myofibrillar effects of levosimendan and enoximone. J Cardiovasc Pharmacol. 2005;46:369–376. doi: 10.1097/01.fjc.0000175454.69116.9. [DOI] [PubMed] [Google Scholar]
- Ukkonen H, Saraste M, Akkila J, Knuuti MJ, Lehikoinen P, Nagren K, et al. Myocardial efficiency during calcium sensitization with levosimendan: a noninvasive study with positron emission tomography and echocardiography in healthy volunteers. Clin Pharmacol Ther. 1997;61:596–607. doi: 10.1016/S0009-9236(97)90139-9. [DOI] [PubMed] [Google Scholar]
- Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, et al. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther. 2000;68:522–531. doi: 10.1067/mcp.2000.110972. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Zhang YY, Gitt A, Belardinelli R, Koike A, Lubarsky L, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96:2221–2227. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. The novel calcium sensitizer levosimendan activates the ATP-sensitive K+ channel in rat ventricular cells. J Pharmacol Exp Ther. 1997;283:375–383. [PubMed] [Google Scholar]
- Zuurbier CJ, Heslinga JW, Lee-de Groot MB, van der Laarse WJ. Mean sarcomere length-force relationship of rat muscle fibre bundles. J Biomech. 1995;28:83–87. doi: 10.1016/0021-9290(95)80009-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


