Figure 4.
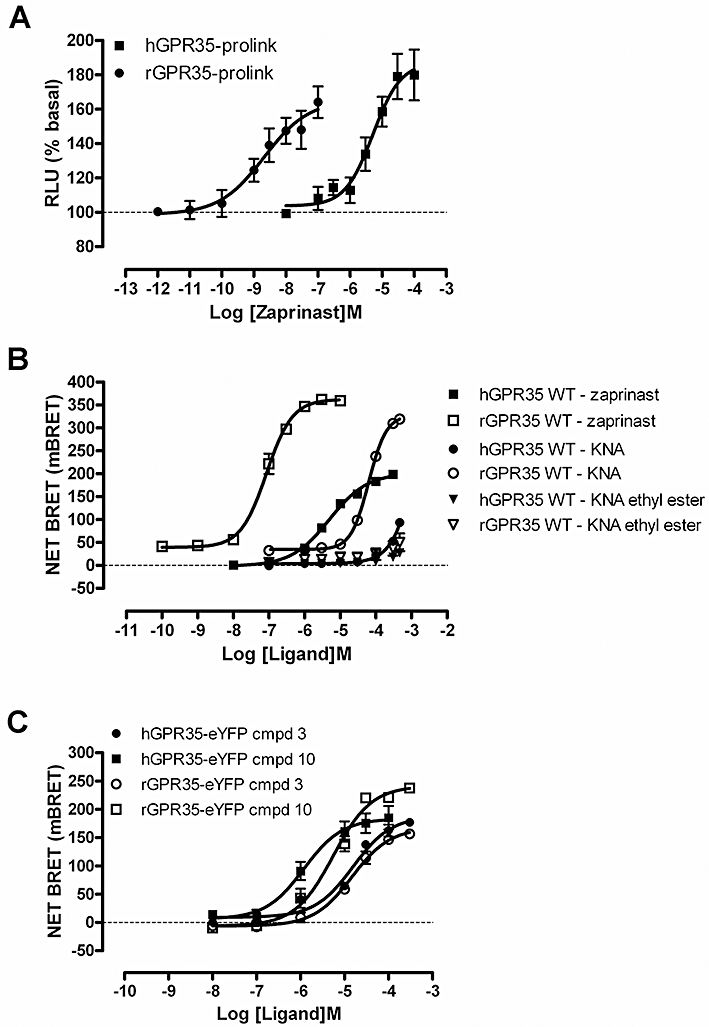
Analysis of GPR35-β-arrestin-2 interactions. (A) Human and rat forms of GPR35 C-terminally tagged with the Prolink™ fragment of β-galactosidase were transiently introduced into HEK 293-BAEA cells stably expressing β-arrestin-2 linked to the remaining element of β-galactosidase. Cells were treated with varying concentrations of zaprinast for 60 min, as suggested by the manufacturer, and β-galactosidase activity reflecting complementation of the enzyme produced via GPR35-β-arrestin-2 interaction was measured. (B) FLAG-hGPR35-eYFP or FLAG-rGPR35-eYFP was co-transfected with β-arrestin-2-Renilla luciferase into HEK293T cells. BRET signals were monitored after treatment of the cells for 5 min with varying concentrations of zaprinast, kynurenic acid or kynurenic acid ethyl ester. (C) Studies akin to those of (B) were conducted using varying concentrations of compound 3 and compound 10 using FLAG-hGPR35-eYFP or FLAG-rGPR35-eYFP.
