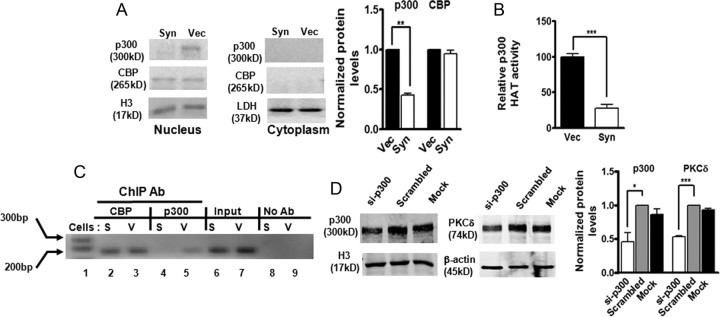
Figure 8.
α-Synuclein downregulates p300 proteins, resulting in decreased p300 HAT activity and inhibition of p300-dependent transactivation of PKCδ gene expression. A, Decreased p300 protein levels in αsyn-expressing cells. Representative immunoblot (left panel) and quantitation (right panel) of p300 and CBP on cytoplasmic and nuclear extracts from αsyn-expressing (Syn) and vector control (Vec) cells. Data are shown as mean ± SEM of two independent experiments (**p < 0.01). LDH (cytoplasmic fraction) or histone H3 (nuclear fraction) was used as loading control. B, Decreased p300 HAT activity in αsyn-expressing cells. Data were subtracted from background values that were measured in samples containing normal IgG, and then expressed as the percentage of HAT activity present in vector control cells. Values are shown as mean ± SEM of three independent experiments performed in triplicate (***p < 0.001). C, The in vivo binding of p300 on the PKCδ promoter was interrupted by overexpression of αsyn. After reversal of cross-linking, p300-immunoprecipitated genomic DNA fragments were analyzed by PCR using primers designed to amplify the −103 to +60 region of PKCδ promoter. D, Knockdown of p300 by siRNA-p300 decreased PKCδ levels in N27 cells. N27 cells were transfected with p300-siRNA and scrambled siRNA for 96 h, and cells were collected for Western blot analysis. Representative immunoblot (left panel) and quantitation (right panel) of p300 and PKCδ on nuclear extracts or whole-cell lysates in transfected cells. Data are shown as mean ± SEM of two independent experiments (*p < 0.05; ***p < 0.001).