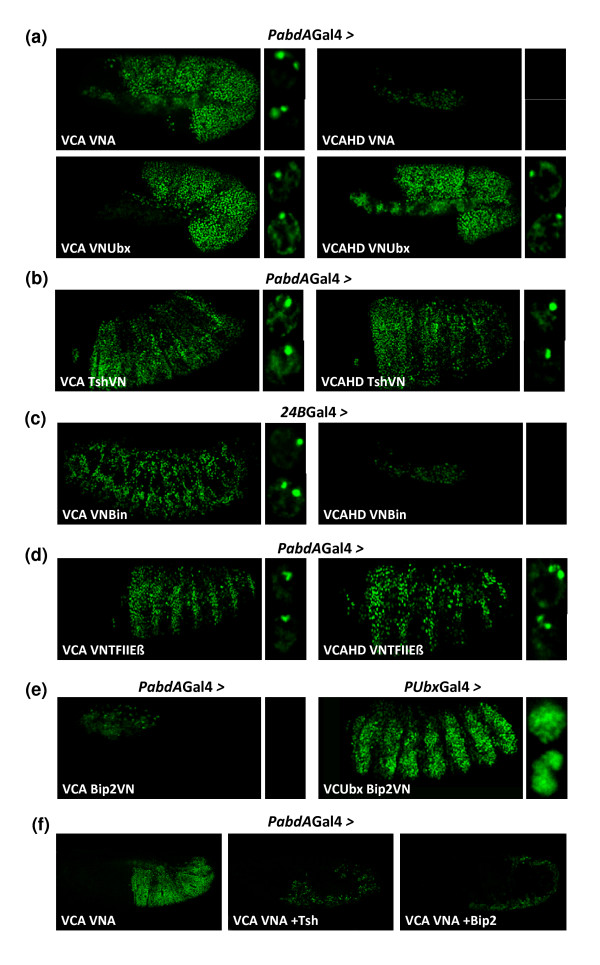
Figure 9.
Suitability of bimolecular fluorescence complementation (BiFC) for in vivo identification of interacting partners (a) BiFC resulting from abdominalA (AbdA)-AbdA and AbdA-ultrabithorax (Ubx) interactions. The homeodomain (HD) mutation in AbdA abolishes homodimeric complex formation only. (b) BiFC between AbdA and the transcription factor Teashirt (Tsh). The HD mutation in AbdA does not abolish AbdA/Tsh complex formation. (c) BiFC between AbdA and the transcription factor Biniou (Bin). Fusions proteins were expressed in the mesoderm. The HD mutation in AbdA abolishes AbdA/Bin complex formation. (d) BiFC between AbdA and the basal transcription machinery protein TFIIbeta. The HD mutation in AbdA does not abolish AbdA/TFIIbeta complex formation. (e) BiFC between AbdA or Ubx and the basal transcription machinery protein BIP2. No BiFC can be visualized between AbdA and BIP2. (f) Competition experiments against BiFC resulting from the VCA/VNA complex assembly. Simultaneous expression of Tsh or BIP2 drastically affects BiFC, suggesting that these two partners interact with AbdA fusion proteins, thereby titrating BiFC complexes. Candidate interacting partners are fused to the VN fragment as indicated, and drivers used are written above pictures. Enlargements on the right focused on the BiFC profile within nuclei.