Table 2. Thermodynamic parameters for scPCNA and hPCNA denaturation by Urea and GuHCl.
| [scPCNA](µM) | [hPCNA](µM) | MidpointDenaturantConcentration(M) |
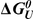 (kcal⋅mol−1) a (kcal⋅mol−1) a
|
m(kcal⋅mol−1⋅M−1) | |
| Urea | 1 | - | 4.3 | 4.8±0.3b | 1.1±0.1 |
| 10 | - | 4.4 | 4.9±0.2b | 1.2±0.1 | |
| 0–2.5 M | - | 1 | 0.8 | 6.5±0.1c | 2.6±0.2 |
| - | 3.4 | 1.4 | 6.50±0.01c | 2.85±0.02 | |
| 2.5–8.8 M | - | 1 | 4.6 | 2.9±0.5d | 1.4±0.2 |
| - | 3.4 | 4.7 | 2.9±0.4d | 1.1±0.1 | |
| GuHCl | 1 | - | 1.4 | 6.97±0.04e | 2.56±0.05 |
| 10 | - | 2.6 | 6.80±0.04e | 2.42±0.04 |
Standard free energy of unfolding at 35°C extrapolated to zero denaturant concentration (See materials and methods). For simplicity all these values are expressed per mol of monomer (unless otherwise specified, see next note).
The two possible mechanisms (data fitting to equations 1 or 2) yield the same numeric result (but per mol of unfolded monomer or per mol of unfolded trimer, respectively). If any of these models is correct, the value would not include the contribution of dissociation.
From equation 4.
From equation 5.
From equation 3.
