Abstract
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy and constitutes 15% of adult leukemias. Although overall prognosis for pediatric ALL is favorable, high-risk pediatric patients and most adult patients have significantly worse outcomes. Multi-agent chemotherapy is standard of care for both pediatric and adult ALL, but is associated with systemic toxicity and long-term side effects, and is relatively ineffective against certain ALL subtypes. Recent efforts have focused on the development of targeted therapies for ALL including monoclonal antibodies. Here we report the identification of CD47, a protein that inhibits phagocytosis, as an antibody target in standard and high-risk ALL. CD47 was found to be more highly expressed on a subset of human ALL patient samples compared to normal cell counterparts and to be an independent predictor of survival and disease refractoriness in several ALL patient cohorts. Additionally, a blocking monoclonal antibody against CD47 enabled phagocytosis of ALL cells by macrophages in vitro, and inhibited tumor engraftment in vivo. Significantly, anti-CD47 antibody eliminated ALL in the peripheral blood, bone marrow, spleen, and liver of mice engrafted with primary human ALL. These data provide pre-clinical support for the development of an anti-CD47 antibody therapy for treatment of human ALL.
INTRODUCTION
Acute lymphoblastic leukemia (ALL), a clonal malignancy of lymphocyte precursors, is the most common malignancy in children, comprising nearly one third of all pediatric cancers and 15% of all de novo leukemias. More than 80% of children diagnosed with ALL can achieve cure with multi-agent treatment regimens (1). In contrast, the prognosis for adults is significantly worse, with a five-year event-free survival (EFS) around 40% (1). Within both pediatric and adult ALL, subsets of patients have significantly worse outcomes with stratification into high-risk categories based upon several criteria including age, initial white blood cell count, presence of extramedullary disease at diagnosis, minimal residual disease, cytogenetic and karyotype analysis, and others (2, 3). In terms of cytogenetic risk, the presence of BCR-ABL (Ph+) or (mixed lineage leukemia) (MLL) rearrangements are associated with an unfavorable prognosis, while the TEL-AML1 rearrangement or trisomy of chromosomes 4, 10, or 17 are more favorable (3). In pediatric cases, high-risk patients have relatively poor prognoses with an estimated four-year EFS of 46% compared to 91% for standard-risk patients (3).
Although multi-agent chemotherapy is mainstay treatment, monoclonal antibodies have emerged as an attractive therapeutic modality due to the ability to selectively target leukemia cells, thereby minimizing systemic toxicity. Indeed, several monoclonal antibodies are currently in clinical trials for the treatment of ALL (reviewed in (4)).
In our previous investigation, we identified CD47 as a therapeutic antibody target in acute myeloid leukemia (AML) (5), and hypothesize that a monoclonal antibody against CD47 could be similarly effective in ALL. As one of several functions, CD47 serves as an inhibitor of phagocytosis by binding its ligand, signal regulatory protein alpha (SIRPα), on phagocytes (6–10). While this function is partly attributed to self-recognition in normal physiologic conditions, many cancers appear to upregulate CD47 as a mechanism of immune evasion (5, 11–13). We have recently demonstrated that this mechanism could be therapeutically targeted in human cancers by a monoclonal blocking anti-CD47 antibody that could eliminate human AML, non-Hodgkin’s lymphoma (NHL), and bladder cancer (5, 12, 13). In the current study, we investigated whether a blocking monoclonal antibody against CD47 could eliminate primary human ALL in vitro and in vivo, in order to determine the pre-clinical feasibility of an anti-CD47 antibody therapy in standard and high-risk ALL.
MATERIALS AND METHODS
Human Samples and Cell Lines
Normal human bone marrow (BM) cells were purchased from AllCells Inc. (Emeryville, CA, USA). Human ALL samples were obtained from patients at the Stanford University Medical Center, with informed consent, according to an IRB-approved protocol (Stanford IRB# 11177). The human T-ALL cell line CCRF-CEM was obtained from the American Type Culture Collection (ATCC) on May 2010 from stock frozen in 2007, characterized by morphology and growth curve analysis by ATCC.
Flow Cytometry Analysis
The following antibodies were used for analysis of ALL and NBM cells: CD3 APC-Cy7 and CD19 APC (BD Biosciences, San Jose, CA, USA). CD47 expression was performed with an anti-human CD47 FITC antibody (clone B6H12.2, BD Biosciences). For human engraftment analysis in mice, antibodies were used as described previously (5).
ALL microarray gene expression data and statistical analysis
We used previously described methods for statistical analyses of CD47 gene expression data and its relationship to clinical variables (5). A detailed description is present in the supplementary methods.
Therapeutic antibodies
Anti-human CD47 antibodies, anti-SIRPα antibody, IgG control, and anti-CD45 antibodies were used as previously described (5). The anti-CD47 antibody clone BRIC126 was obtained from AbD Serotec (Raleigh, NC, USA).
Generation of mouse and human macrophages
Isolation of mouse and human macrophages were performed as previously described (5).
In vitro phagocytosis assays
Phagocytosis assays were performed as described (5). Briefly, bulk ALL cells were CFSE-labeled and incubated with either mouse or human macrophages in the presence of 10 µg/ml of the indicated antibodies at a target:effector cell ratio of 4:1 (2×105:5×104).
Ex vivo antibody coating of ALL cells
Human ALL cells were incubated with 30 µg/ml of either IgG1 isotype control, anti-CD45, or anti-CD47 antibody for 30 minutes at 4°C. Cells were washed and then 1–4×106 cells were transplanted into sublethally-irradiated NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) adults or pups and analyzed for ALL engraftment in the peripheral blood (PB) and BM 6–10 weeks later. Antibody coating of ALL cells was confirmed by flow cytometry with a secondary antibody prior to transplantation into mice. Sublethal irradiation was given at 230 rads and 100 rads for NSG adults and pups, respectively.
In vivo treatment of human ALL engrafted mice
1–4×106 bulk human ALL cells were transplanted intravenously via retro-orbital sinus into sublethally-irradiated adult NSG mice or into the facial vein of 2–4 day old sublethally-irradiated NSG pups. Six weeks later, PB and BM ALL engraftment (B-ALL: hCD45+CD19+; T-ALL: hCD45+CD3+) was assessed by tail bleed and aspiration of the femur, respectively. Engrafted mice were treated for 14 days with daily 100 µg intraperitoneal injections of either IgG control or anti-CD47 antibody (clone B6H12.2). On day 15, mice were sacrificed and analyzed for ALL engraftment in the PB, BM, spleen, and liver. For treatment of CCRF-CEM engrafted mice, daily 200 µg injections of either IgG or anti-CD47 antibody was administered for 12 days unless otherwise indicated.
Bone marrow tissue section preparation and staining
Mouse tibias from antibody-treated NSG mice were harvested and preserved in formalin. Hematoxylin and eosin staining and immunohistochemistry of human CD45+ cells were performed by Comparative Biosciences Inc. (Sunnyvale, CA, USA).
Generation of a luciferase-positive CCRF-CEM cell line and in vivo imaging
CCRF-CEM cells were transduced with lentivirus encoding the dual reporter gene L2G (Luc-2A-eGFP) as previously described (13). Adult NSG mice were transplanted intravenously into the tail vein with 2×106 luciferase-labeled CCRF-CEM cells. Post-transplant day 5, mice were treated with daily injections of IgG or anti-CD47 antibody for the indicated times. Bioluminescent imaging was performed as previously described (13).
Secondary Transplants of AML-engrafted mice
Human ALL-engrafted mice were treated with daily 200µg intraperitoneal injections of anti-CD47 antibody or IgG control for 14 days. 2×106 bulk BM cells from anti-CD47 antibody or IgG treated mice post-treatment were transplanted into sublethally-irradiated NSG adults. AML engraftment of secondary transplants was assessed six weeks later by analysis of human CD45+ chimerism in the BM by flow cytometry.
RESULTS
CD47 Expression is Increased on a Subset of Human ALL Cells Compared to Normal Bone Marrow
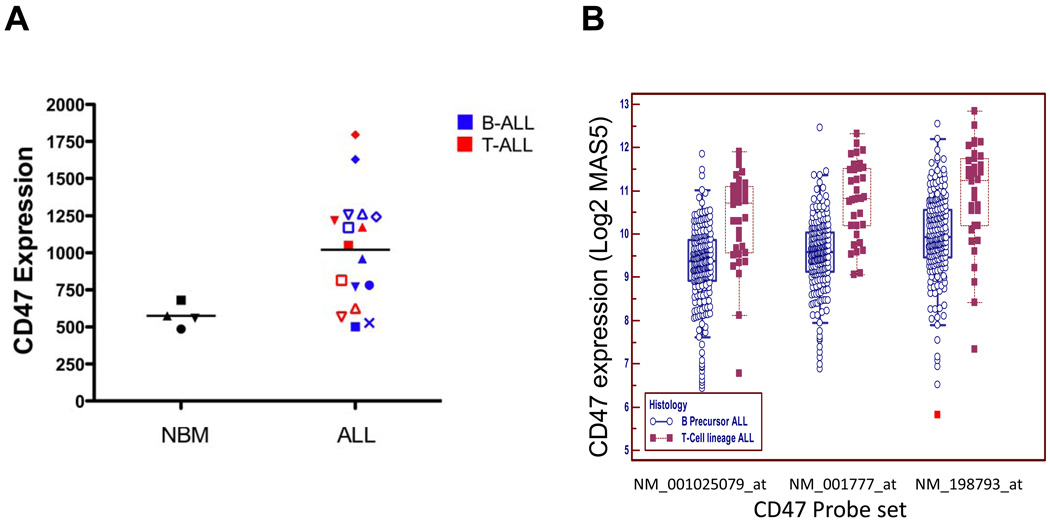
We first investigated CD47 cell surface expression on primary human ALL and normal BM cells by flow cytometry. We surveyed 17 diverse patients with ALL that included both precursor B and T lineage subtypes (Figure 1A, Supplementary Table S1). Compared to normal mononuclear BM cells, CD47 was more highly expressed on human ALL samples, approximately 2-fold when considering all samples, with similar expression between B and T subtypes (Figure 1A,B). However, assessing CD47 mRNA expression in a previously described large cohort of ALL patients (14), we found that T-ALL patients expressed significantly higher levels compared to B-ALL patients (Figure 1B).
Figure 1. CD47 expression is increased on a subset of human ALL cells compared to normal bone marrow.
(A) Relative CD47 protein expression was determined on normal human BM cells and human ALL cells (Supplementary Table S1) by flow cytometry. CD47 expression was increased on bulk ALL cells compared to normal BM cells (p=0.006). Within all samples, normalized mean expression (and range) was determined as follows: NBM 575.3 (486.6–680.9), bulk ALL 1020.2 (501.3 – 1794.5), B-ALL 980.5 (501.3 – 1628.4), and T-ALL 1034.9 (626.2 – 1794.5). Differences between mean expression of NBM and T-ALL (p=0.03), NBM and B-ALL (p=0.006) were statistically significant. (B) Relative CD47 mRNA expression was determined in a large cohort of ALL patients (n=254) from (14) and subdivided into B or T-ALL subtypes. Using three different probe sets, CD47 expression was higher in T-ALL patients compared to B-ALL patients (p<0.0001 for all three probe sets).
CD47 Expression is an Independent Prognostic Predictor in Mixed and High-Risk ALL
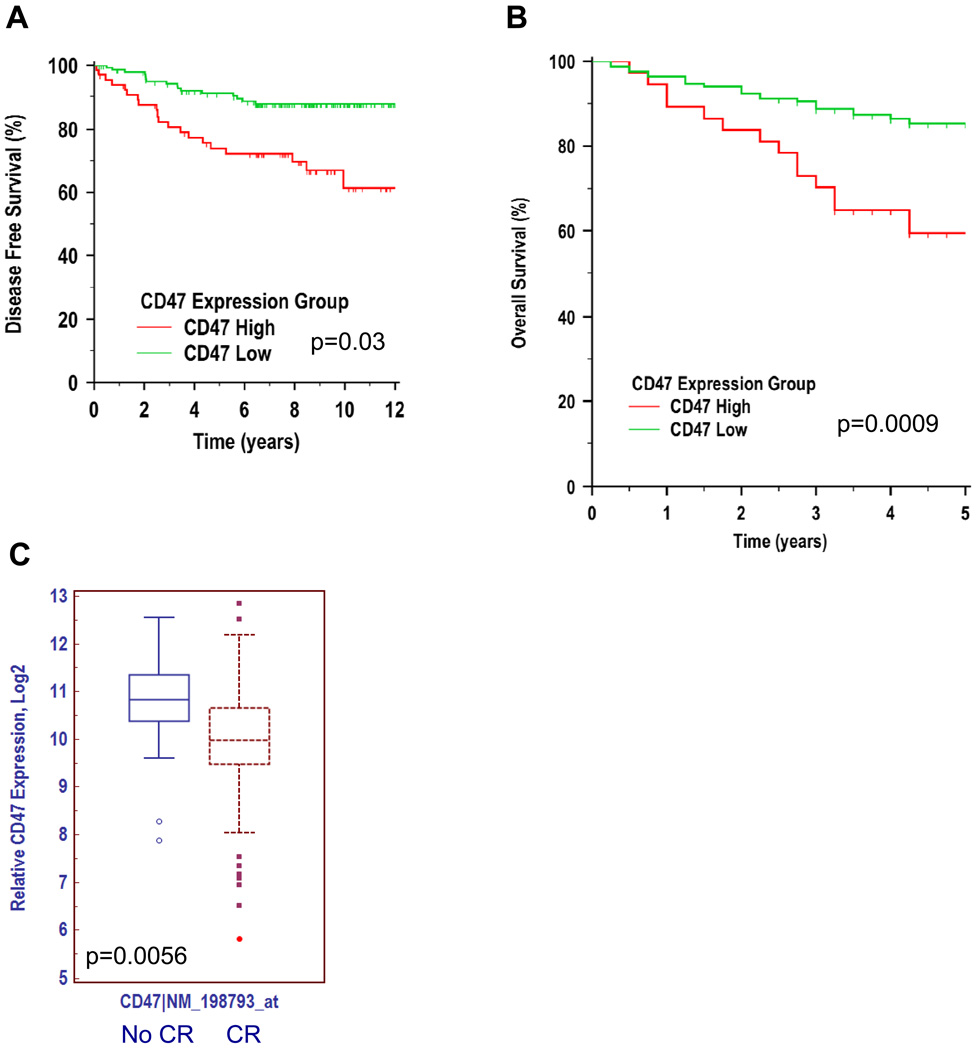
Since CD47 expression was increased on ALL samples, with observed heterogeneity in CD47 expression across ALL subtypes, we investigated whether the level of CD47 expression correlated with clinical prognosis. First, CD47 expression was investigated as a prognostic predictor in pediatric ALL patients with mixed risk and treatment utilizing gene expression data from a previously described patient cohort (15). This diverse risk cohort included patients with BCR-ABL rearrangements, MLL rearrangements, hyperdiploidy, hypodiploidy, as well as both B- and T-ALL subtypes. 360 patients were stratified into high and low CD47-expressing groups based on an optimal cutpoint (see methods) and clinical outcomes were determined. Among the subset of this cohort with available outcome data (n=205) (16), patients expressing higher levels of CD47 had worse outcomes, whether CD47 expression was tested as a continuous variable (p=0.03; hazard ratio (HR) 1.78 per 2-fold change in CD47 expression; 95% confidence interval (CI) 1.05–3.03), or as a dichotomous variable relative to an internally validated optimal threshold (uncorrected p=0.0005, corrected (17) p=0.01; HR 3.05; 95% CI 1.49– 6.26) (Figure 2A and Supplementary Table S2A).
Figure 2. CD47 expression is an independent prognostic predictor in mixed and high-risk ALL.
(A) Pediatric ALL patients (n=360) with mixed risk and treatment (15) were stratified into CD47 high- and low-expressing groups based on an optimal cut point. Disease-free survival (DFS) was determined by Kaplan-Meier analysis (see Supplementary Table S2A). (B) Pediatric ALL patients (n=207) with high-risk (defined by age>10 years, presenting WBC count>50,000/µl, hypodiploidy, and BCR-ABL positive disease) and uniform treatment (18) were stratified into CD47 high- and low-expressing groups using a similar approach as in A (see Supplementary Table S2B). (C) CD47 expression was analyzed on ALL patients from a third cohort (14), stratified into groups either achieving a complete remission (CR) or not (no CR).
We next investigated the prognostic power of CD47 expression in high-risk ALL patients, specifically in a cohort of 207 patients, uniformly treated, with high risk defined by age>10 years, presenting WBC count>50,000/µl, and central nervous system (CNS) or testicular involvement (18). Higher CD47 expression correlated with a worse overall survival when considered as either a continuous variable (p=0.0009, HR 3.59 per 2-fold change in CD47 expression; 95% CI 1.70 to 7.61), or a dichotomous variable relative to an internally validated optimal threshold (uncorrected p=0.001, corrected p=0.01; HR 2.80; 95% CI 1.21 to 6.50) (Figure 2B and Supplementary Table S2B). In multivariate analysis, CD47 expression remained a significant prognostic factor when age at diagnosis, gender, WBC count, CNS involvement, and minimal residual disease were considered as covariates (Supplementary Table S3).
Lastly, we utilized a third independent gene expression dataset to investigate whether CD47 expression could predict refractoriness to primary treatment (14). Indeed, CD47 expression was higher in uniformly treated patients failing to achieve a complete remission (CR) compared to those that did (Figure 2C). Taken together, these observations among distinct and diverse cohorts establish that higher expression of CD47 is an independent predictor of adverse outcomes in pediatric patients with standard- and high-risk ALL, including induction failure, refractory disease, and death.
Blocking Monoclonal Antibodies Against CD47 Enable Phagocytosis of ALL Cells
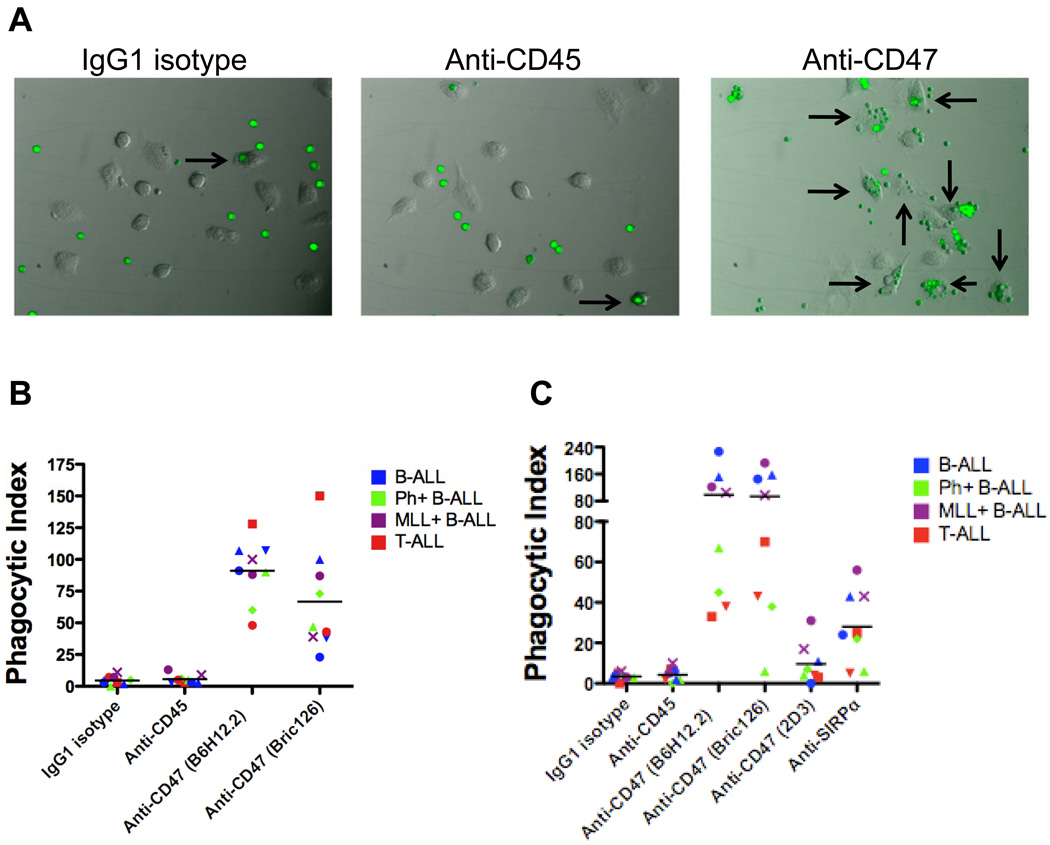
Next, we investigated whether ALL cells could be eliminated by macrophage phagocytosis enabled through blockade of the CD47-SIRPα interaction. We incubated human macrophages with fluorescently-labeled ALL cells in the presence of an IgG1 isotype control, anti-CD45 isotype-matched, or blocking anti-CD47 antibody and measured phagocytosis by fluorescence microscopy (Figure 3A). Two different blocking anti-CD47 antibodies (B6H12.2 and BRIC126) enabled phagocytosis of ALL cells compared to IgG1 isotype and anti-CD45 control antibodies as measured by phagocytic index (Figure 3B). Notably, anti-CD47 antibodies enabled phagocytosis of all ALL subtypes profiled, including those with high-risk cytogenetic abnormalities (Ph+ALL and MLL+ALL). Since several studies report that CD47-SIRPα signaling may be species-specific (19, 20), the ability of anti-CD47 antibody-coated human cells to be phagocytosed by mouse macrophages was determined before proceeding with in vivo antibody treatment experiments in mouse xenotransplants. Similarly, two blocking anti-CD47 antibodies (B6H12.2 and BRIC126) enabled increased phagocytosis of ALL cells by mouse macrophage effectors compared to IgG1 isotype and anti-CD45 antibody controls (Figure 3C). In contrast, no phagocytosis was observed with a non-blocking anti-CD47 antibody (2D3). Lastly, blockade of SIRPα with an anti-mouse SIRPα antibody also resulted in increased phagocytosis, thus supporting the proposed mechanism of increased phagocytosis resulting from disruption of the CD47-SIRPα interaction (Figure 3C).
Figure 3. Blocking monoclonal anti-CD47 antibodies enable phagocytosis of ALL cells by human and mouse macrophages in vitro.
(A) Primary human ALL cells were fluorescently-labeled and incubated with human macrophages in the presence of the indicated antibodies for 2 hours and then examined by fluorescence microscopy (Leica) with Image-Pro Plus software. Representative photomicrographs are shown with ALL cells (green) and arrows indicating macrophages containing phagocytosed ALL cells. (B) The phagocytic index (number of target cells ingested per 100 macrophages) was determined for the indicated antibodies. The anti-CD47 antibodies B6H12.2 and BRIC126 enabled significant levels of phagocytosis compared to IgG1 isotype or anti-CD45 antibody controls (p<0.0001). (C) The in vitro phagocytosis assay was conducted using mouse macrophages as effector cells. Compared to IgG1 isotype and anti-CD45 antibody controls, the blocking anti-CD47 antibodies B6H12.2 and BRIC126 (p<0.0001) and anti-mouse SIRPα antibody (p=0.002) enabled phagocytosis of ALL cells. In contrast, the non-blocking anti-CD47 antibody, 2D3, did not enable phagocytosis compared to IgG1 isotype (p=0.17). Each data point represents an ALL patient sample as labeled in Figure 1C. Statistical comparisons were conducted with a two-sided student t-test.
Ex Vivo Coating of ALL Cells with an Anti-CD47 Antibody Inhibits Leukemic Engraftment
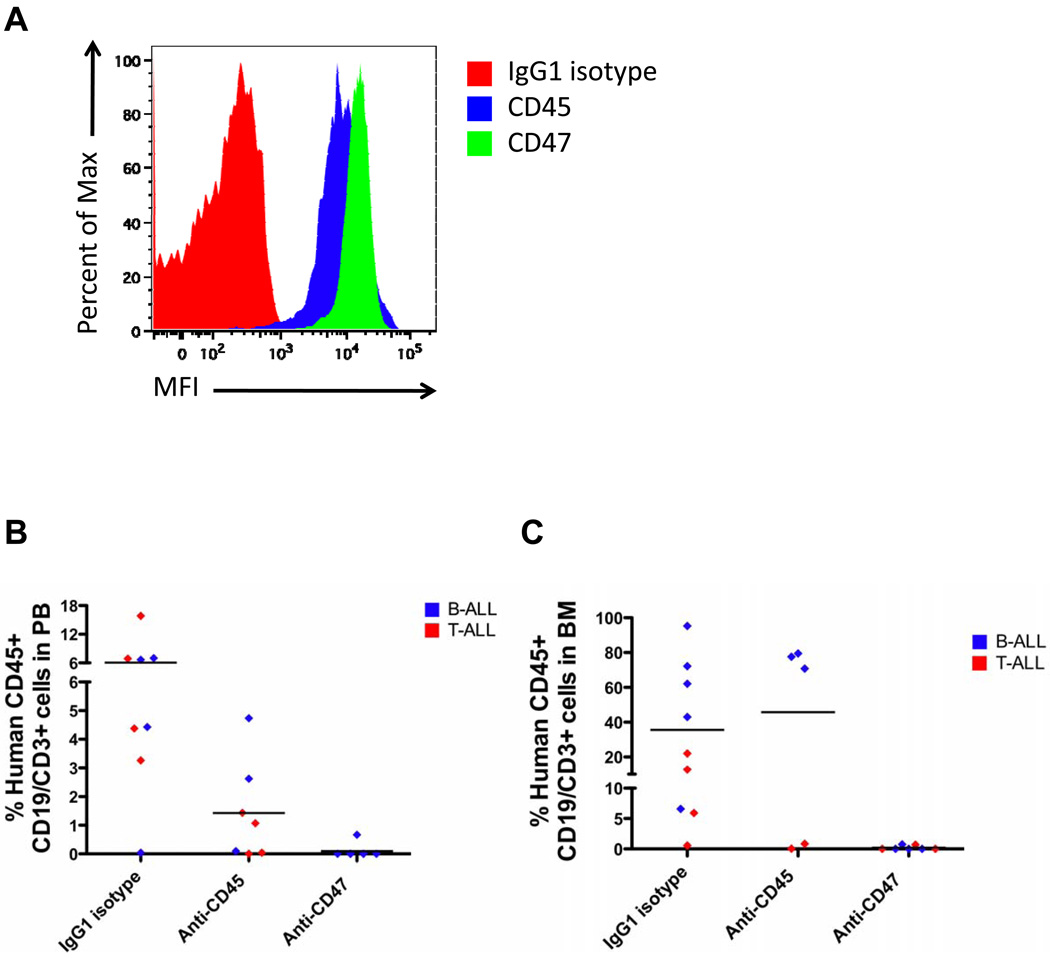
The ability of a blocking anti-CD47 antibody to eliminate ALL in vivo was investigated by two independent methods. First, the anti-CD47 antibody was assessed for inhibition of both B- and T-ALL engraftment using an antibody pre-coating assay. ALL cells were coated ex vivo with either IgG1 isotype control, anti-CD45, or anti-CD47 antibody (B6H12.2), transplanted into sublethally-irradiated immunodeficient NOD/SCID/IL2Rγ null (NSG) mice, and measured for ALL engraftment in the PB and BM 6 weeks later. Prior to transplantation, coating of ALL cells with antibody was verified by flow cytometry (Figure 4A). Anti-CD47 antibody significantly inhibited leukemic engraftment of both B- and T-ALL cells in the PB (Figure 4B) and BM (Figure 4C) compared to IgG1 isotype or anti-CD45 antibody controls. Interestingly, pre-coating of T-ALL cells (but not B-ALL cells) with anti-CD45 antibody reduced tumor engraftment. Anti-CD45-mediated inhibition of T-ALL engraftment was unlikely due to antibody-opsonization, given that B-ALL cells coated with anti-CD45 antibody engrafted similarly to uncoated cells incubated with IgG1 isotype control antibody. Rather the effect observed in T-ALL may be due to modulation of CD45-dependent functions important to engraftment for T-ALL but not B-ALL. Regardless, pre-coating with the anti-CD47 antibody nearly completely eliminated ALL engraftment in vivo.
Figure 4. Ex vivo coating of ALL cells with an anti-CD47 antibody inhibits leukemic engraftment.
(A) ALL cells were incubated with the indicated antibodies in vitro with antibody coating detected by a fluorescently-labeled secondary antibody (representative plot shown). (B–C) Pre-coated ALL cells were transplanted into NSG mice, and human ALL engraftment was assessed 6 weeks later in the PB (B) or BM (C) by flow cytometry. Ex vivo coating of ALL cells (ALL4 [red] and ALL8 [blue]; Supplementary Table S1) with anti-CD47 antibody inhibited engraftment compared to IgG1 isotype control in the PB (p=0.02) and BM (p=0.02). No difference in engraftment levels was detected between anti-CD45 antibody and IgG1 isotype control (p=0.67, considering both B and T-ALL samples). Each symbol represents a different primary ALL sample, with each point representing a different mouse. p-values were calculated using the Fisher’s exact test.
Anti-CD47 Antibody Eliminates ALL Engraftment in the Peripheral Blood and Bone Marrow
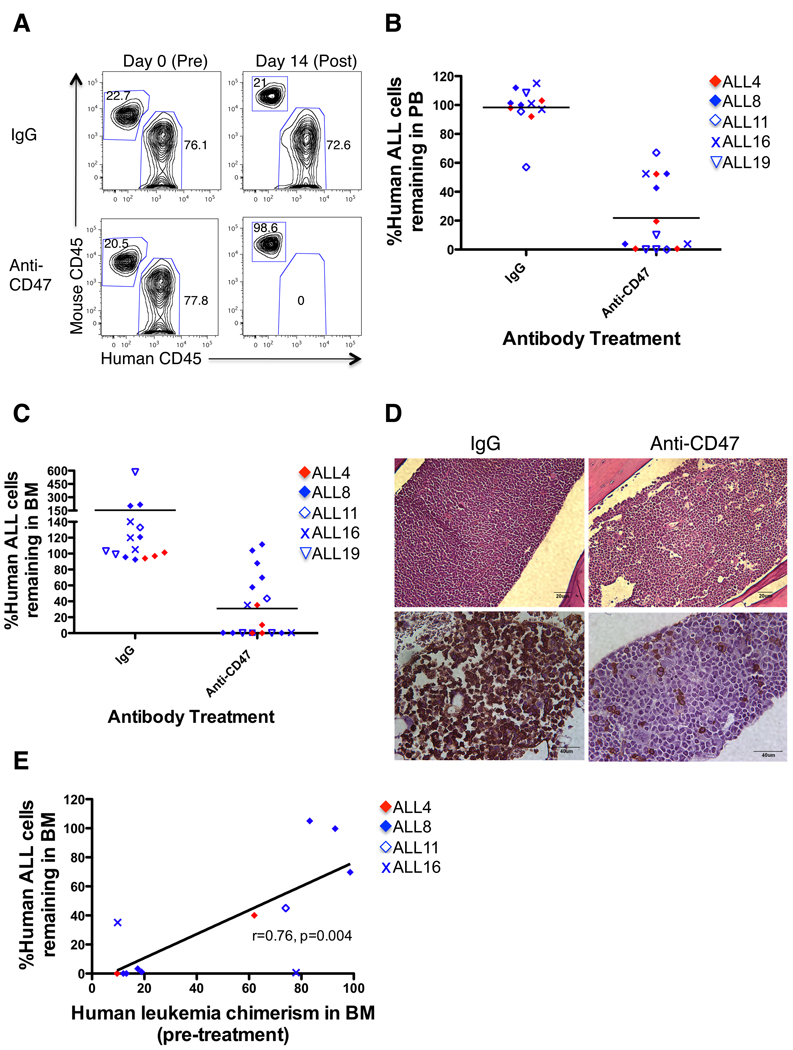
In the second method of investigating anti-CD47 antibody efficacy in vivo, mice were first engrafted with ALL cells and then treated with antibody. B- or T-ALL cells were transplanted into NSG mice with leukemic disease analyzed in the PB or BM six weeks later. Mice with significant levels of ALL engraftment (greater than 10% leukemia in the PB and/or BM; range=10–98% [data not shown]) were then selected for in vivo antibody therapy (Figure 5A). ALL engrafted mice were treated with daily intraperitoneal injections of 100 µg IgG control or anti-CD47 antibody (B6H12.2) for 14 days as determined by our prior in vivo studies in AML and NHL (5, 13). Compared to IgG, anti-CD47 antibody therapy reduced the level of circulating leukemia, and in many cases eliminated B- or T-ALL from the blood (Figure 5A,B) or BM (Figure 5C). BM histology of antibody-treated mice revealed infiltration of monomorphic leukemic blasts in control IgG-treated mice (Figure 5D). ALL-engrafted mice treated with anti-CD47 antibody exhibited normal mouse hematopoietic cells with cleared hypocellular areas in the BM (Figure 5D). Immunohistochemistry of mouse marrows confirmed near complete invasion of human CD45-positive leukemic blasts in IgG-treated marrow compared to few human CD45-positive leukemia cells detected in anti-CD47 antibody-treated marrow (Figure 5D). In some cases, anti-CD47 antibody therapy had a minimal effect on reducing leukemia in the BM (Figure 5C, solid blue diamond symbols). We attribute this relative lack of efficacy to the extremely high levels of leukemic engraftment in the BM of these mice, where it is likely that an insufficient number of host macrophages were present to mediate phagocytic elimination of leukemic cells. Indeed, a positive correlation was observed between the percentage of human ALL cells remaining after anti-CD47 antibody treatment and the level of pre-treatment leukemic burden in the BM (Figure 5E).
Figure 5. Anti-CD47 antibody eliminates ALL engraftment in the peripheral blood and bone marrow.
(A) NSG mice engrafted with primary B and T-ALL patient samples were treated with IgG or anti-CD47 antibody. PB human ALL chimerism pre- and post-treatment was measured by flow cytometry (representative mice shown). (B) Anti-CD47 antibody treatment reduced the level of circulating leukemia compared to IgG when all samples were pooled (p<0.0001) or when mice were analyzed by individual ALL sample (ALL4: p=0.01, ALL8: p=0.01, ALL16: p=0.03, ALL19: p=0.001). (C) Anti-CD47 antibody treatment reduced ALL engraftment in the BM compared to IgG when all samples were pooled (p=0.007) or when analyzed by individual ALL sample (ALL4: p=0.001, ALL8: p=0.009, ALL16: p=0.01, ALL19: p=0.16). Each symbol represents a different patient sample, with each point representing a different mouse. Statistical analysis on ALL11-engrafted mice was not performed due to insufficient sample number. (D) BM sections from hematoxylin and eosin staining (top) and immunohistochemical human CD45 staining (bottom) are shown from representative mice engrafted with B-ALL post-treatment. (E) The correlation between pre-treatment disease burden and disease remaining post-anti-CD47 antibody treatment was analyzed by Pearson’s correlation.
Anti-CD47 Antibody Eliminates ALL Engraftment in the Spleen and Liver
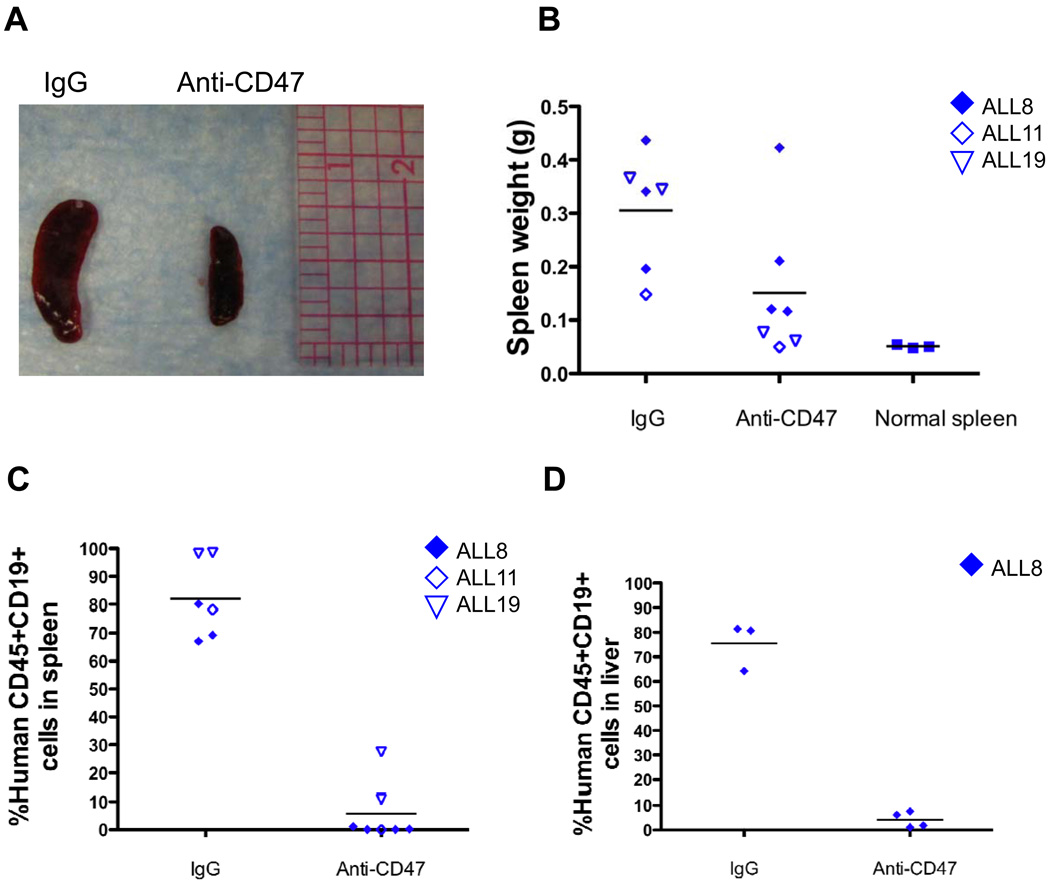
Hepatomegaly and splenomegaly can cause clinical complications and are a common finding in ALL, being observed in up to 69% of patients at diagnosis (21, 22). Accordingly, we investigated the ability of anti-CD47 antibody to eliminate ALL engrafted in the spleen and liver. We identified three B-ALL patient samples (ALL8, ALL21, and ALL22) that gave rise to disease in the spleen and/or liver, with associated splenomegaly, when transplanted into NSG mice. Control IgG-treated B-ALL-engrafted mice exhibited significant splenomegaly compared to untransplanted NSG mice (Figure 6A,B). In contrast, anti-CD47 antibody treatment reduced splenomegaly to spleen sizes similar to untransplanted NSG mice (Figures 6A,B). To determine whether this effect was due to direct elimination of ALL cells in the spleen, these spleens were then analyzed for ALL disease burden. Compared to IgG-treated mice, anti-CD47 antibody eliminated B-ALL in the spleen (Figure 6C). Similarly, anti-CD47 antibody eliminated ALL in the liver compared to extensive leukemic infiltration observed with control IgG treatment (Figure 6D). These results indicate that anti-CD47 antibody is highly effective in eliminating ALL in the spleen and liver, in addition to the PB and BM.
Figure 6. Anti-CD47 antibody eliminates ALL engraftment in the spleen and liver.
(A) Representative spleens are shown from NSG mice engrafted with cells from ALL mice treated with IgG or anti-CD47 antibody. (B) Spleen weights from anti-CD47 antibody-treated mice were reduced compared to IgG-treated mice (p=0.04) to sizes similar to that of normal NSG spleens (p=0.09). IgG-treated mice exhibited splenomegaly compared to normal mice (p=0.0002). (C–D) Levels of ALL engraftment were determined post-antibody treatment in the spleen (C) and liver (D) by flow cytometry. Compared to IgG, anti-CD47 antibody treatment eliminated ALL in the spleen (p<0.0001) and liver (p<0.0001).
Anti-CD47 Antibody Treatment Induces Remissions in ALL Engrafted Mice
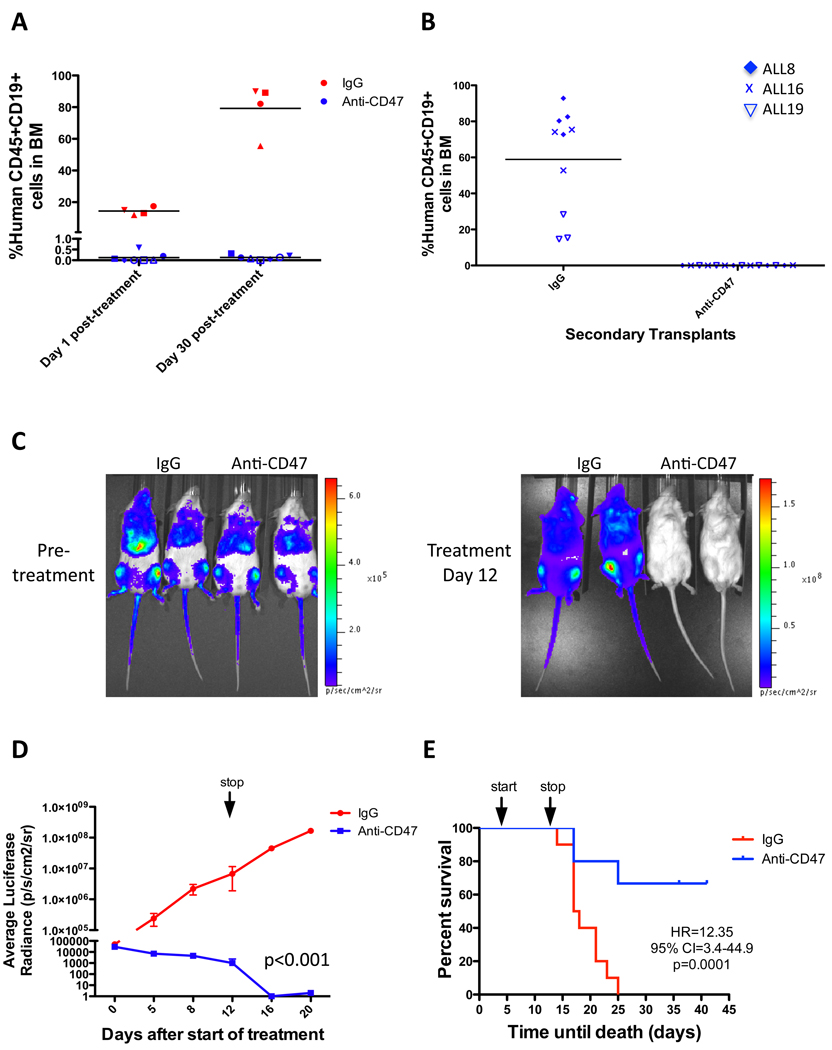
We next investigated whether tumor elimination by anti-CD47 antibody was maintained long-term using three independent methods. First, B-ALL engrafted mice with equivalent levels of leukemia were treated with antibody therapy for 2 weeks with BM analyzed for leukemic disease immediately post-treatment. Consistent with prior experiments, anti-CD47 antibody eliminated leukemic disease compared to IgG controls (Figure 7A). Treatment was then stopped and BM was analyzed four weeks later to assess disease levels. While control IgG treated mice exhibited expansion of leukemic disease, anti-CD47 antibody treated mice had no evidence of disease or relapse (Figure 7A). In the second method, secondary transplants were performed by transplanting BM from previously treated mice to determine whether anti-CD47 antibody treatment could eliminate ALL tumorigenic potential. Secondary mice transplanted with control IgG treated BM cells exhibited robust ALL engraftment in the BM while mice transplanted with cells from anti-CD47 antibody treated mice did not engraft, indicating that anti-CD47 antibody eliminated ALL tumorigenicity (Figure 7B). Third, to investigate the long-term efficacy of anti-CD47 antibody in T-ALL, the human T-ALL cell line CCRF-CEM was engineered to express luciferase and transplanted into NSG mice for antibody treatment. Mice were serially monitored for luciferase positive leukemia by bioluminescent imaging and survival during and after antibody therapy (Figure 7C). Anti-CD47 antibody treatment markedly reduced leukemic disease compared to control IgG treatment (Figure 7C,D). After treatment was stopped, leukemic disease disseminated in control IgG treated mice leading to death by 25 days post-transplant. In contrast, over 60% of anti-CD47 antibody-treated mice exhibited long- term survival (Figure 7E) with no evidence of relapse in surviving mice (Figure 7D). These experiments demonstrate that anti-CD47 antibody treatment is able to induce long-term clearance of ALL in vivo.
Figure 7. Anti-CD47 antibody treatment induces remissions in ALL engrafted mice.
(A) Leukemic BM chimerism is shown for mice engrafted with ALL8 either 1 day or 30 days after completion of antibody therapy. Day 1 post-treatment, leukemic disease was eliminated in anti-CD47 antibody treated mice compared to IgG controls (p=0.001). Thirty days post-treatment, anti-CD47 antibody treated mice showed no evidence of relapse (p=0.68), while leukemic burden increased in IgG controls (p<0.001) in comparison to day 1 post-treatment. Each symbol represents the same mouse 1 day and 30 days post-treatment. (B) Leukemic engraftment is shown from secondary mouse recipients transplanted with equal numbers of BM cells from mice treated with either IgG or anti-CD47 antibody. BM cells from IgG controls engrafted robustly in secondary recipients, while cells from anti-CD47 antibody treated mice did not engraft (p<0.0001, Fisher’s exact test). Each data point represents a different mouse transplanted with the indicated ALL sample. (C) Luciferase-expressing CCRF-CEM cells were transplanted into NSG mice and analyzed for engraftment by bioluminescent imaging on day 5 post-transplant (pre-treatment) and end of treatment (day 12) with representative mice shown. (D) Luciferase signal was quantified across all mice (n=5 per treatment) demonstrating reduction in luciferase positive leukemia with anti-CD47 antibody treatment compared to IgG control (p<0.001, 2 way ANOVA). *Three mice died in the IgG cohort on treatment day 12, with subsequent luciferase measurements obtained in the remaining mice. (E) Kaplan-Meier analysis was performed with identical treatment conditions as in D (Mantel-Cox test was used to calculate HR and 95% CI). Mice sacrificed due to significant disease-related morbidities were included as disease-related deaths. N=10 in each treatment group. Arrows represent start and stop of antibody treatment.
DISCUSSION
We report here that CD47 is expressed at high levels on a large subset of human ALL subtypes, is an independent prognostic predictor in ALL for survival and disease refractoriness in both mixed and high-risk ALL patients, and is a monoclonal antibody target for elimination of ALL blasts through macrophage-mediated phagocytosis. Together, these data suggest that ALL pathogenesis relies on mechanisms to evade innate immune recognition and that modulation of the innate immune recognition of leukemia cells may be a viable treatment modality.
CD47 is broadly expressed on hematopoietic cells and other normal tissues (23), which could potentially lead to toxic effects with an anti-CD47 antibody therapy. Despite this expression, we have previously demonstrated that administration of anti-CD47 antibody to normal cells including human CD34+ BM precursors and PB cells in vitro does not lead to phagocytic engulfment (5, 13). Also, administration of a blocking anti-mouse CD47 antibody to wild type mice causes minimal toxicity, principally an isolated neutropenia (5). This lack of toxicity is likely not entirely due to CD47 expression level, as anti-CD47 antibody equally coated both normal and leukemia cells at the therapeutic dose administered. Alternatively, the phagocytosis stimulated by anti-CD47 antibody may be due to an imbalance of pro- and anti-phagocytic signals on leukemic blasts with expression of an as yet uncharacterized positive stimulus for phagocytosis present on leukemia cells but not normal cells. Such candidate stimuli include phosphatidylserine (24), annexin-1 (25), and calreticulin (26) which are targets under active investigation.
Within the last several years, several cell surface proteins have been identified as therapeutic targets with monoclonal antibodies proceeding into early and late phase clinical trials. Most therapeutic antibodies in clinical development have been focused on B-ALL. One candidate is CD20, based on its expression in approximately 40 to 50% of B-ALL cases (reviewed in (27)). Rituximab, an anti-CD20 antibody, initially approved for treatment of B cell lymphoma, has demonstrated a significant survival advantage when added to standard chemotherapy in some ALL clinical trials, particularly against the Burkitt’s subtype (28, 29). Although effective in adult CD20+ B-ALL, there is a paucity of clinical data on the efficacy of rituximab in pediatric ALL. In contrast to CD20, CD22 is expressed in a larger percentage of B-ALL cases, being present on greater than 90% of B-ALL. Epratuzumab, a humanized monoclonal anti-CD22 antibody, is currently being investigated with early clinical studies in relapsed ALL showing limited effect as a single agent (30). However, anti-CD22 antibody-immunotoxin conjugates are being explored in Phase I trials (31, 32), since CD22 is reported to be rapidly internalized upon antibody binding (33). In addition, antibodies and immunotoxins to other antigens including CD19 are currently being explored ((34) (reviewed in (35)).
Perhaps the best success of targeted therapy has been observed in Ph+ B-ALL. Since its demonstration of efficacy in chronic myeloid leukemia, imatinib, an ABL tyrosine kinase inhibitor, has been utilized in Ph+ B-ALL with some success. As a single agent, imatinib can produce response rates of 20–30%; however, these response durations are short (36). The combination of imatinib with chemotherapy has been more promising, with three-year overall survival rates of 55% in patients treated with imatinib+hyperCVAD compared to 15% for patients receiving hyperCVAD alone (37).
In contrast to B-ALL, there are few antibody therapies being investigated for treatment of T-ALL. The most prominent antibody for T-ALL, alemtuzumab, is targeted at CD52, which is expressed on greater than 95% of normal lymphocytes and at higher levels on T compared to B lymphoblasts (38). However, early phase clinical trials do not report a significant benefit as a single agent or in combination with chemotherapy for the treatment of relapsed T-ALL (39).
In contrast to the targeted therapies developed for B-ALL and T-ALL, our data provides a strong pre-clinical rationale that an anti-CD47 antibody can be effective in eliminating both B- and T-ALL as well as high-risk ALL.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Adriane Mosley for animal husbandry and Libuse Jerabek and Theresa Storm for excellent lab management. M.P.C. is supported by grants from the Howard Hughes Medical Institute and Stanford University Cancer Biology Program. R.M. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. This research is supported by funds from the Ludwig Institute via the Ludwing Center at Stanford University and from NIH grant CA139490. M.P.C., R.M., and I.L.W. filed US Patent Application Serial No. 12/321,215 entitled “Methods For Manipulating Phagocytosis Mediated by CD47.”
REFERENCES
- 1.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 2.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov. 2007;6:149–165. doi: 10.1038/nrd2240. [DOI] [PubMed] [Google Scholar]
- 5.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blazar BR, Lindberg FP, Ingulli E, et al. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med. 2001;194:541–549. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazawa H, Motegi S, Ohyama N, et al. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174:2004–2011. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 8.Oldenborg PA, Zheleznyak A, Fang YF, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 9.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med. 2001;193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willman CL. Discovery of novel molecular classification schemes and genes predictive of outcome in leukemia. Hematol J. 2004;5 Suppl 3:S138–S143. doi: 10.1038/sj.thj.6200440. [DOI] [PubMed] [Google Scholar]
- 15.Yeoh EJ, Ross ME, Shurtleff SA, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 16.Holleman A, den Boer ML, Cheok MH, et al. Expression of the outcome predictor in acute leukemia 1 (OPAL1) gene is not an independent prognostic factor in patients treated according to COALL or St Jude protocols. Blood. 2006;108:1984–1990. doi: 10.1182/blood-2006-04-015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller R, Siegmund D. Maximally selected chi square statistics. Biometrics. 1982;38:1011–1016. [Google Scholar]
- 18.Kang H, Chen IM, Wilson CS, et al. Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia. Blood. 115:1394–1405. doi: 10.1182/blood-2009-05-218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takenaka K, Prasolava TK, Wang JC, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRPalpha. Blood. 2006;107:2548–2556. doi: 10.1182/blood-2005-04-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Till MM, Hardisty RM, Pike MC. Long survivals in acute leukaemia. Lancet. 1973;1:534–538. doi: 10.1016/s0140-6736(73)90342-5. [DOI] [PubMed] [Google Scholar]
- 22.Onciu M, Lai R, Vega F, Bueso-Ramos C, Medeiros LJ. Precursor T-cell acute lymphoblastic leukemia in adults: age-related immunophenotypic, cytogenetic, and molecular subsets. Am J Clin Pathol. 2002;117:252–258. doi: 10.1309/08DJ-GPBH-H0VR-RC6F. [DOI] [PubMed] [Google Scholar]
- 23.Reinhold MI, Lindberg FP, Plas D, et al. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J Cell Sci. 1995;108(Pt 11):3419–3425. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- 24.Fadok VA, Voelker DR, Campbell PA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 25.Scannell M, Flanagan MB, deStefani A, et al. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–4605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- 26.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Jeha S. New therapeutic strategies in acute lymphoblastic leukemia. Semin Hematol. 2009;46:76–88. doi: 10.1053/j.seminhematol.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 29.Gokbuget N, Hoelzer D. Novel antibody-based therapy for acute lymphoblastic leukaemia. Best Pract Res Clin Haematol. 2006;19:701–713. doi: 10.1016/j.beha.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study. J Clin Oncol. 2008;26:3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrera L, Bostrom B, Gore L, et al. A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2009;31:936–941. doi: 10.1097/MPH.0b013e3181bdf211. [DOI] [PubMed] [Google Scholar]
- 32.Wayne AS, Kreitman RJ, Findley HW, et al. Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin Cancer Res. 16:1894–1903. doi: 10.1158/1078-0432.CCR-09-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du X, Beers R, Fitzgerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uckun FM, Messinger Y, Chen CL, et al. Treatment of therapy-refractory B-lineage acute lymphoblastic leukemia with an apoptosis-inducing CD19-directed tyrosine kinase inhibitor. Clin Cancer Res. 1999;5:3906–3913. [PubMed] [Google Scholar]
- 35.Gokbuget N, Hoelzer D. Treatment with monoclonal antibodies in acute lymphoblastic leukemia: current knowledge and future prospects. Ann Hematol. 2004;83:201–205. doi: 10.1007/s00277-003-0752-8. [DOI] [PubMed] [Google Scholar]
- 36.Ottmann OG, Wassmann B. Imatinib in the treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia: current status and evolving concepts. Best Pract Res Clin Haematol. 2002;15:757–769. doi: 10.1053/beha.2002.0233. [DOI] [PubMed] [Google Scholar]
- 37.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 38.Alinari L, Lapalombella R, Andritsos L, et al. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene. 2007;26:3644–3653. doi: 10.1038/sj.onc.1210380. [DOI] [PubMed] [Google Scholar]
- 39.Angiolillo AL, Yu AL, Reaman G, et al. A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: a Children's Oncology Group report. Pediatr Blood Cancer. 2009;53:978–983. doi: 10.1002/pbc.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.