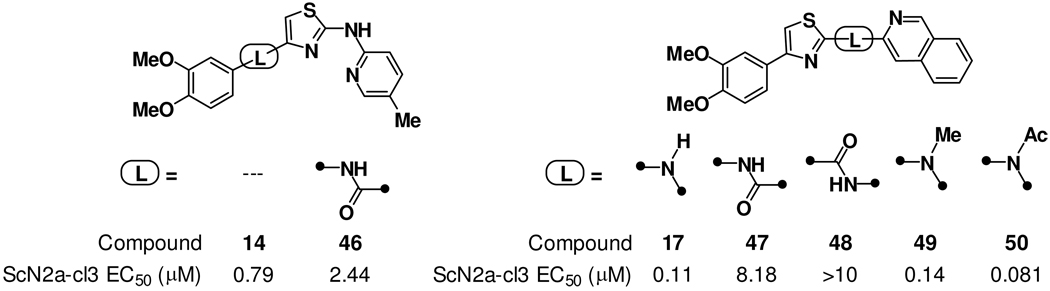
Figure 4.
Chemical structures and antiprion activity of analogs with modified A–B ring linkages (46) or B–C ring linkages (47–50). The introduction of an amide linkage was better tolerated at the A–B ring connection (46) than at the B–C ring connection (47 and 48). Alkylation or acylation of the amino B–C ring linkage was tolerated (17 vs 49 and 50) demonstrating that a hydrogen bond donor is not required in this position.