Figure 1.
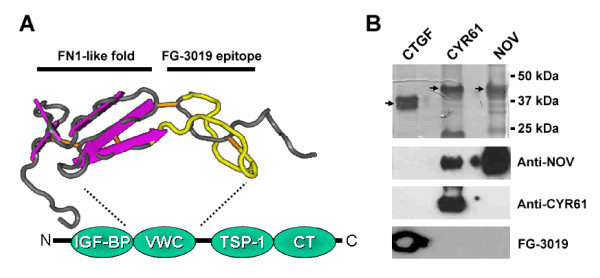
The FG-3019 binding epitope overlaps with the VWC domain of connective tissue growth factor (CTGF). (A) The region of the FG-3019 binding epitope (CTGF amino acids 142 to 157, indicated in yellow) is superimposed on the structure of the collagen IIa VWC domain [50]. By sequence homology, the general structural features of this domain are predicted to be conserved in CTGF and other CCN (connective tissue growth factor) proteins [51]. The predicted FG-3019 binding site lies outside of a fibronectin-1-like module within the VWC domain of CTGF. A physical interaction between the VWC domain of Xenopus CTGF and TGF-β family members TGF-β1 and bone morphogenetic protein (BMP)-4 was reported [9]. The location of the VWC domain relative to the insulin-like growth factor binding protein (IGF-BP), thrombospondin-1 (TSP1) and C-terminal cysteine knot (CT) homology domains of CCN family members is also indicated. (B) Recombinant human CTGF, CYR61 and NOV proteins (visualized by Coomassie blue in the upper panel) were analyzed by western blot using FG-3019, anti-CYR61 and anti-NOV antibodies. FG-3019 specifically bound CTGF without crossreacting with CYR61 or NOV.