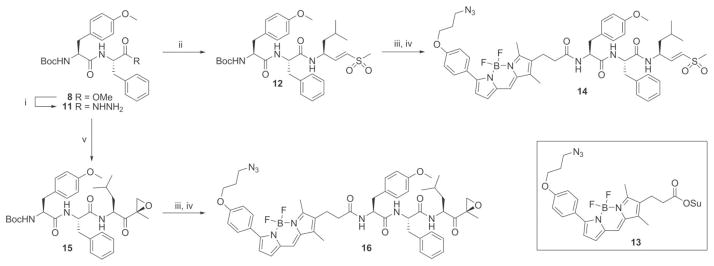
Scheme 2.
Synthesis of azido-BODIPY probes 14 and 16. Reagents and conditions: (i) Hydrazine monohydrate (60 equiv.), MeOH, reflux, 2 h, 88%. (ii) (a) HCl (2.8 equiv.), tBuONO (1.1 equiv.), DMF–EtOAc (1: 1, v/v), −25 °C, 4 h. (b) TFA·H-LeuVS (1.1 equiv.), DiPEA (3.8 equiv.), −25 °C to RT, 15 h, 75%. (iii) TFA/DCM 1: 1 (v/v), 30 min. (iv) 13 (1 equiv.), DiPEA (1 equiv.), DCM, 15 h, 14 54%, 16 27%. (v) (a) HCl (2.8 equiv.), tBuONO (1.1 equiv.), DMF–EtOAc (1: 1, v/v), −25 °C, 4 h. (b) TFA·H-LeuEK (1.1 equiv.), DiPEA (3.8 equiv.), −25 °C to RT, 15 h, 71%.