Figure 1.
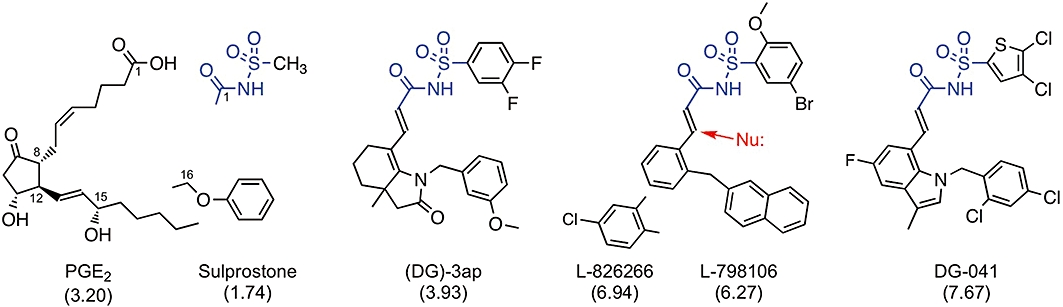
Structures of EP3 receptor ligands: prostaglandin E2 (PGE2) and its analogue sulprostone are agonists; the other compounds are antagonists. All compounds are weak acids: proton loss from carboxylate group or acyl-sulphonamide unit (blue). Predicted n-octanol-water partition coefficients (AlogP98) are shown in parentheses; differences between the antagonists are mainly due to the (lower) acyl moieties. The red arrow indicates potential covalent attack on the α, β-unsaturated amide group by a nucleophile (Nu:).
