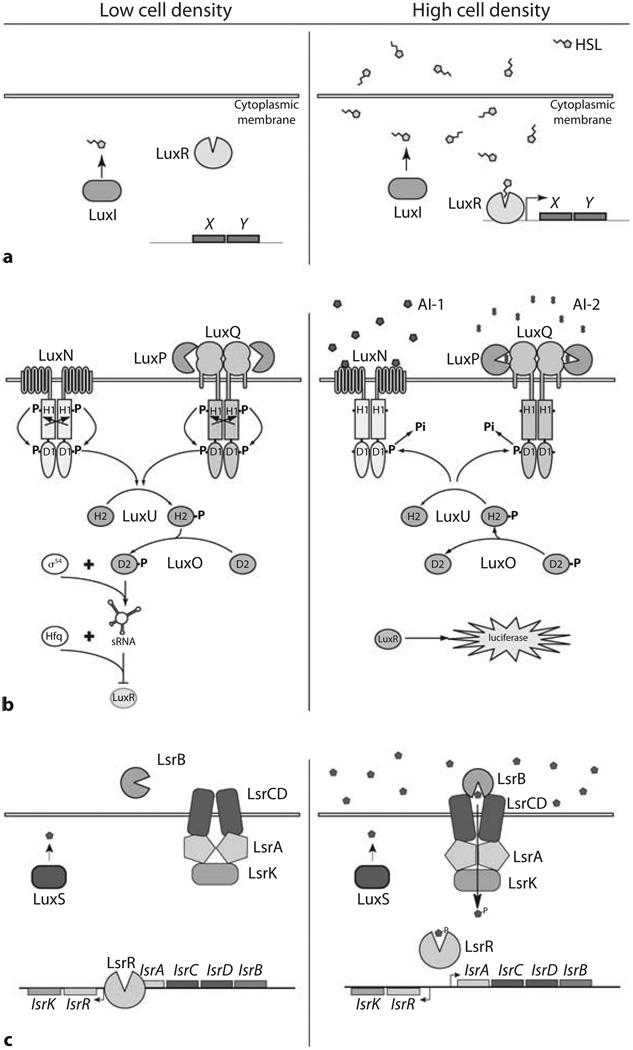
Fig. 1.
Quorum-sensing signaling networks. Low cell density (left sides of panels), High cell density (right sides of panels). a A LuxI/LuxR quorum-sensing circuit. The LuxI enzyme produces an AHL molecule that diffuses into the surroundings. At high cell densities, the LuxR protein binds the AHL and subsequently binds to DNA promoting the transcription of genes X and Y. b V. harveyi AI-1- and AI-2-dependent circuits. At low cell density, LuxN and LuxQ exist in kinase mode autophosphorylating at histidine (H1) and aspartic acid (D1) residues. Phosphate is passed to LuxU at the histidine (H2) site, and then to LuxO at an aspartic acid (D2). LuxO-P, with sigma factor σ54, activates transcription of the sRNA genes. The sRNAs, with the RNA chaperone Hfq repress luxR expression. At high cell densities, AI-1 binds LuxN and AI-2 binds LuxP-LuxQ. Binding of autoinducers causes LuxN and LuxQ to switch to phosphatase mode. Phosphate is drained from LuxU and LuxO, terminating expression of the sRNA genes. LuxR is derepressed, and luciferase is expressed. c The E. coli and S. typhimurium Lsr system. At low cell densities, LsrR binds to DNA and represses expression of the lsr operon. At high cell densities, AI-2 is imported via the Lsr transporter and is phosphorylated by LsrK. AI-2-phosphate binds to LsrR causing it to release DNA, thus derepressing lsr transcription [adapted from 8, 28].