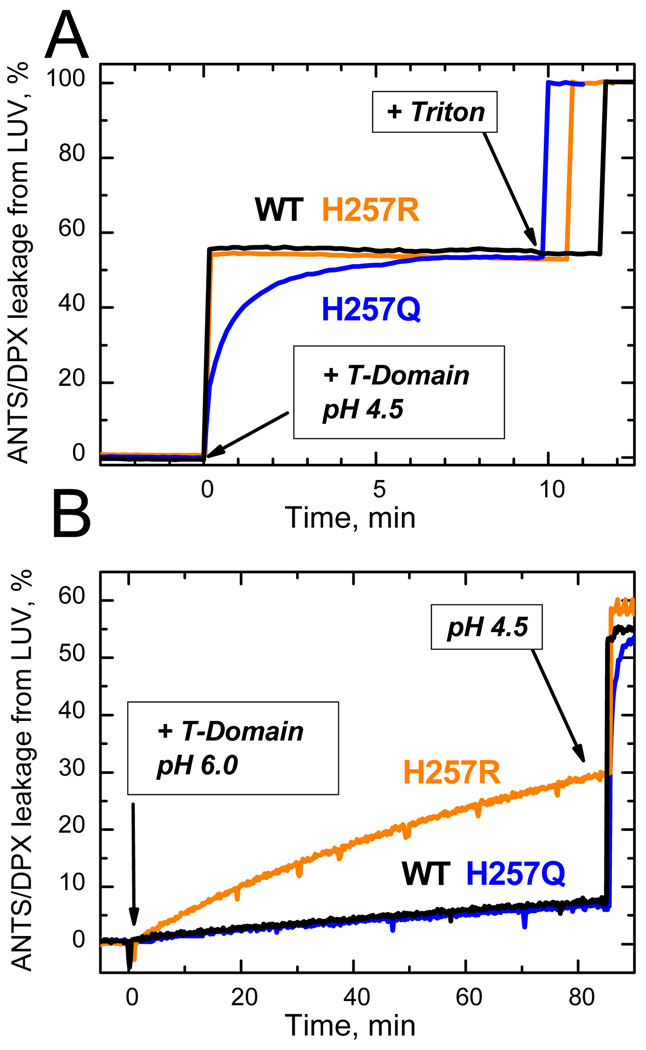
Figure 3.
Leakage of lipid vesicle contents resulting from membrane insertion of the T-domain WT and H257R and H257Q mutants, monitored by increase of fluorescence from ANTS/DPX-loaded LUV. Membrane interactions were initiated at zero time by adding stocks of T-domain to vesicular samples at pH 4.5 and 6.0. Both mutants demonstrated a similar level of membrane-disrupting activity at pH 4.5, although the kinetics of membrane action was slower in H257Q (A). Correspondingly, H257R showed substantially greater membrane disruption at pH 6.0 than did WT or H257Q (B). The traces for WT measured at pH 6.0 and 4.5 coincide with those for the respectively neutral and charged replacements of H257, indicating that the protonation of this residue in WT is responsible for triggering its pH-dependent membrane action. Samples contained 0.2 µM T-domain and 25% POPC/75% POPG LUV containing 0.2 mM of total lipid. Other experimental details are described in 17.