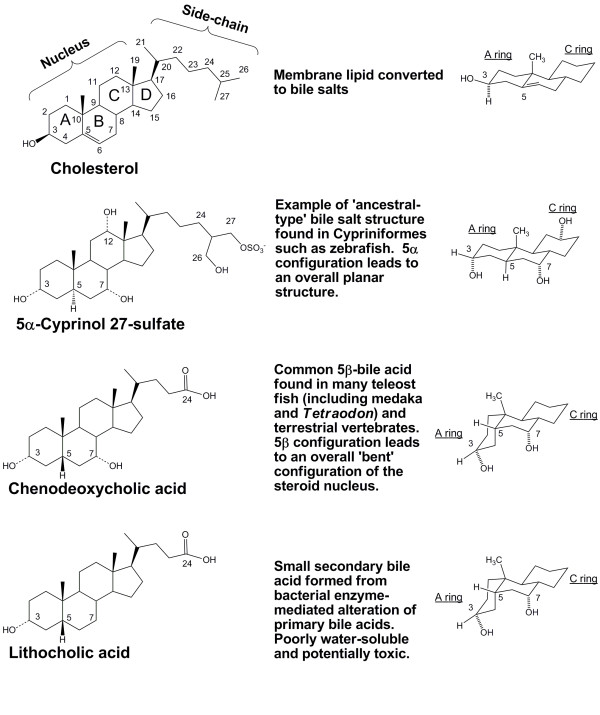
Figure 1.
Representative bile salts and their structures. All bile salts are derived from cholesterol (topmost structure), illustrated with the carbon atoms numbered and the steroid rings labelled A, B, C, and D. Jawless fish, lobe-finned fish, and a limited number of actinopterygian fish use 5α bile alcohols such as 5α-cyprinol-27-sulfate that have an overall planar and extended structure of the steroid rings (see representation of A, B, and C rings on the right side). Most actinopterygian fish (including medaka and Tetraodon nigrivirdis) use 5β bile acids that have an overall bent structure of the steroid rings. One of the two most common primary salts in mammals is chenodeoxycholic acid (CDCA), the stem C24 bile acid that has the basic 3α,7α-dihydroxylation pattern. Lithocholic acid is one of the smallest naturally occurring bile acids and results from bacterial enzyme-mediated deconjugation and dehydroxylation of primary bile acids. The sodium and calcium salts of lithocholic acid have very low solubility at body temperature. Additionally, lithocholic acid is toxic in humans and other mammals.