Figure 6.
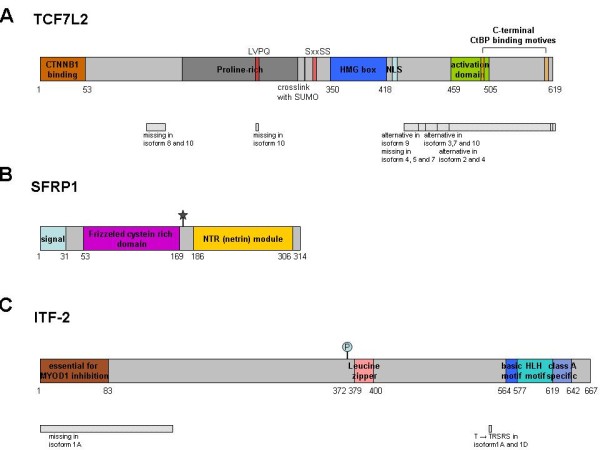
Schematic representation of TCF7L2, SFRP1 and ITF-2. (A) TCF7L2 can be divided in 3 major domains: the β-catenin binding domain (CTNNB1), the DNA-binding HMG box and a C-terminal domain which is variable in many isoforms. Only the long form is able to bind to the COOH-terminal binding protein (CtPB), the C-terminus has an important regulatory function. The presence of the CtBP motif is required for the possible repressor function of TCF7L2. Many splicing variants (>16) have been described and each cell expresses more than one variant [32,37]. (B) SFRP1 is a secreted protein and contains a frizzled typical cysteine rich domain (Fz-CRD) which displays the putative Wnt-ligand binding site. The C-terminal part of the protein shows homology to netrin (NTR), an extracelluar protein involved in axonal guidance. NTR domains are involved in protein binding and contain segments rich of positively charged residues. This region is reported to bind to heparin [34]. (C) ITF-2 binds to DNA via its basic-helix-loop-helix (bHLH) domain and can be regulated by β-catenin. The Pitt-Hopkins syndrome (PTHS), a rare syndromic encephalopathy, is caused by ITF-2 haploinsufficency. At least 3 isoformal sequences have been described. The two major isoforms, ITF-2A and ITF-2B, have different N-termini resulting from alternative promoter usage [30]. (⋆ = glycosilation, p = phosphorylation. Swissprot accession numbers Q9NQB0, Q8N474, P15884)
