Abstract
Objective
For at least three decades, many investigators have reported on the US black/white breast cancer case ratio for estrogen receptor (ER) status as if it reflected an intrinsic biological difference. In light of racial/ethnic differences in declines in the incidence of ER+ breast cancer, as linked to changing use of hormone therapy, we empirically tested whether the black/white breast cancer estrogen receptor ratio has changed over time.
Methods
We examined temporal trends in the odds of being ER+ among white as compared to black women among all cases of invasive breast cancer occurring among women residing in the catchment area of the SEER 13 Registries Database between 1992 and 2005.
Results
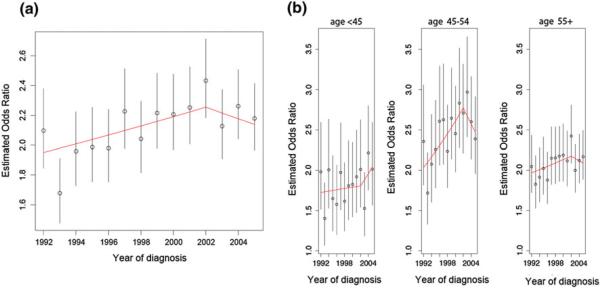
During the study period, the odds of being ER+ among the white compared to black cases increased from 1992 to 2002 (a statistically significant joinpoint; p < 0.05; peak odds ratio (2002) = 2.25 (95% confidence interval 2.13, 2.39)). Thereafter, the odds ratio leveled off (post-2002 slope not significantly different from zero; p = 0.326). Among women aged 45–54, moreover, the post-2002 decline tended toward statistical significance (p = 0.0891).
Conclusions
The results suggest the black/white breast cancer case estrogen receptor ratio is historically contingent, not innate.
Keywords: Black, Breast cancer, Cancer disparities, Estrogen receptor, Social determinants of health, White
Introduction
For at least three decades, many investigators have reported on the US black/white breast cancer case ratio for estrogen receptor (ER) status as if it reflected an intrinsic biological difference [1–5]. Suggesting it is timely to revisit this assumption, however, are two considerations. The first is new data on changes in the incidence of ER-positive (ER+) breast cancer as linked to declines in the use of hormone therapy [6, 7], following the 2002 publication of the Women's Health Initiative (WHI) study, which found no benefit of hormone therapy for preventing cardiovascular disease while reconfirming elevated risk for breast cancer [8]. The second concerns well-known US racial/ethnic and socioeconomic disparities in use of hormone therapy, whereby use has been highest among white and more affluent women [9, 10]. The net implication is that changes in hormone therapy use would be likely result in changes in observed racial/ethnic differences in ER+ status among women with breast cancer.
Supporting the inference that the social patterning of ER status among breast cancer cases might change over time, we recently have reported that post-WHI declines in US breast cancer incidence rates were evident only among non-Hispanic white women living in affluent counties who had ER+ tumors [11]. Here, we present ancillary findings on the temporal trends, among the cases, for the odds of being ER+ among the non-Hispanic white as compared to black women diagnosed with breast cancer.
Materials and methods
The study base consisted of all cases of primary invasive breast cancer among women residing in the catchment area of the public access SEER 13 Registries Database [12] from January 1, 1992 through December 31, 2005 and included 261,476 white non-Hispanic cases and 30,093 black non-Hispanic cases. Use of these de-identified public access county-level cancer registry data was approved as exempt by the Harvard School of Public Health Human Subjects Committee (HSC protocol #P14605-101).
Analytically, the first step was to calculate, for each year, the percent of observed ER+ tumors among the women, stratified by race/ethnicity and age, and compute the age-standardized percent, using the year 2000 standard million [13]. We additionally calculated the age-standardized 3-year average annual percent, for women of all ages and also stratified by age (<45, 45–54, and 55+ years old), for three time points: the start of the study period (1992–1994), the years immediately preceding and including publication of the WHI (2000–2002), and the post-WHI period (2003–2005). We then employed logistic regression models using a linear spline approach with a fixed joinpoint of 2002 [14], set at when the WHI was published, to model the temporal trend in the non-Hispanic white/black odds ratio for being ER+, using the yearly ER data and including age at diagnosis and cancer registry as covariates. We deliberately restricted these analyses to cases for whom ER data were available, so as to replicate the analytic approach used in prior studies on the black/white odds ratio for ER status [1–5]; in our discussion, we consider the implications of missing data for our results.
Results
As shown in Fig. 1a, during the study period (1992–2005), the odds of being ER+ among the white compared to black cases increased from 1992 to 2002 (a statistically significant joinpoint; p < 0.05), with the peak odds ratio in 2002 equal to 2.25 (95% CI 2.13, 2.39) (fitted estimate based on the joinpoint model). Thereafter, the white/black odds ratio leveled off (post-2002 slope not significantly different from zero; p = 0.326). As shown by Fig. 1b, this trend was most pronounced among women aged 45–54 years old (post-2002 decline in the odds ratio tending toward statistical significance; p = 0.0819). By contrast, among women age 55 and older and also under age 45, the post-2002 decline in the odds ratio was not statistically significant (p values of 0.4633 and 0.4177, respectively).
Fig. 1.
Odds ratio for breast cancer cases that are estrogen receptor positive (ER+), adjusted for age and cancer registry, comparing non-Hispanic white to non-Hispanic black women: a all ages; b stratified by age: <45, 45–54, ≥55: US SEER 13 Registries Database (1992–2005)
Driving these temporal change in the odds ratio were changes in the percent of ER+ tumors, which varied by time period, race/ethnicity, and age (Table 1). Among both the non-Hispanic white and non-Hispanic black women in all three age groups (<45, 45–54, and 55+ years old), the percent increased from 1992 to 1994 through 2000–2002 (except among non-Hispanic black women under age 45, for whom it remained stable). Thereafter, the percent of ER+ tumors either leveled off or decreased slightly in each of these groups of women.
Table 1.
Total number (n) and average annual age-standardized percent of primary invasive breast cancer cases that are estrogen receptor positive (ER+) among non-Hispanic white women (n = 261,476) and non-Hispanic black women (n = 30,093), overall and by age: US SEER 13 Registries Database (1992–2005)
| Years | All ages |
<45 years old |
45–54 years old |
55+ years old |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White |
Black |
White |
Black |
White |
Black |
White |
Black |
|||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| 1992–1994 | 30,318 | 63 | 2,265 | 53 | 2,940 | 50 | 395 | 44 | 5,218 | 70 | 456 | 53 | 22,160 | 81 | 1,414 | 67 |
| 1995–1999 | 60,695 | 68 | 4,666 | 53 | 5,599 | 58 | 757 | 45 | 11,914 | 74 | 1,066 | 55 | 43,182 | 82 | 2,843 | 69 |
| 2000–2002 | 40,227 | 75 | 3,312 | 52 | 3,633 | 70 | 528 | 43 | 8,079 | 77 | 780 | 56 | 28,515 | 84 | 2,004 | 70 |
| 2003–2005 | 39,206 | 73 | 3,966 | 52 | 3,705 | 66 | 634 | 43 | 8,375 | 77 | 935 | 57 | 27,126 | 82 | 2,397 | 69 |
Age standardized to the Year 2000 standard million
Discussion
Our study, based on US national cancer registry data, is the first to quantify and test for temporal trends in the odds ratio for comparisons of the observed ER status among black and white women with breast cancer. Results indicate that between 1992 and 2005, the odds for being ER+ among US non-Hispanic white as compared to black women diagnosed with breast cancer increased until 2002 and then leveled off, especially among women aged 45–54. These changes are temporally consistent with reported patterns of hormone therapy use preceding and then following publication of the WHI in 2002, whereby usage before the WHI was highest among newly menopausal and postmenopausal white and/or more affluent women, and with only women who used hormone therapy before the WHI being eligible to stop using hormone therapy after the WHI [9, 10]. Additional research, from epidemiologic to basic science, has provided robust evidence that use of hormone therapy increases risk of ER+ breast cancer tumors [6, 7, 15].
A distinguishing feature of our study is our focus on temporal trends in the black/white difference in the observed ER status among women diagnosed with breast cancer. Most studies comparing the black/white odds among breast cancer cases for being ER+ typically have focused on cases diagnosed in a given year or in a small set of years, with results nonetheless often interpreted as if they constituted a time-invariant intrinsic racial/ethnic difference [1–5]. Yet, as demonstrated by other research on temporal changes in the magnitude of health inequities, both the size and direction of socioeconomic as well as racial/ethnic disparities in health can change over time [16, 17]. Well-known examples include the class shift in smoking, and hence, smoking-related diseases, from professionals to the working class over the course of the twentieth century in wealthy countries [18, 19], and also the US white/black reversal of breast cancer mortality rates, whereby in the mid-20th century, the age-adjusted breast cancer mortality rate was higher among white women, but by the mid-1980s was higher among black women [20, 21].
We further note that we specifically restricted our study to analysis of only cases with observed ER status, so that our results could be compared to prior studies of the black/white odds ratio for ER status, which used data only on observed cases and did not take into account the impact of missing ER data [1–5]. In prior research, however, we have demonstrated that the black/white odds ratio for being ER− is biased upwards when analyses do not take into account the greater prevalence of missing data on ER status among women who live in less-affluent census tracts; we additionally have shown that the age-standardized statistically significant excess in risk of ER status unknown among black compared to white women was rendered statistically non-significant by controlling for socioeconomic position [22]. In our study of racial/ethnic and socioeconomic differentials in the decline of breast cancer incidence following publication of the WHI [11] (using the same case data as employed in this current study), we consequently included analysis of trends in the incidence of tumors with ER status unknown, as well as for tumors that were ER+ and ER−. Relevant to interpretation of our results for trends in the white/black odds ratio in ER+ tumors, we found that these incidence rates, for breast cancer with ER status unknown, were (1) among both the black and white women highest among women age 65 and older, followed by the women aged 50–69, but negligible among those under age 50, and also that (2) the socioeconomic gap, among women age 50 and older, was greater among the black as compared to white women [11]. The net implication is to suggest our present study's findings likely underestimate the magnitude of temporal changes in the white/black odds ratio for ER+ tumors.
In summary, our results provide novel evidence that the black/white breast cancer case estrogen receptor ratio is historically contingent, not innate. The larger implication is that research on racial/ethnic disparities involving tumor biology—and health status more generally—should not automatically assume that observed differences are fixed and reflect intrinsic biology. Instead, also meriting consideration is the alternative hypothesis, supported by considerable evidence, that societal conditions shape the expression of observed biological characteristics and hence the existence of—and trends in—the magnitude of health inequities [16, 17, 23–25].
Acknowledgments
This project was funded by the National Institutes of Health (grant 5R03CA132131 to NK).
References
- 1.Natrajan N, Nemoto T, Mettlin C, Murphy GP. Race-related differences in breast cancer patients: results of the 1982 National Survey of Breast Cancer by the American College of Surgeons. Cancer. 1985;56:1704–1709. doi: 10.1002/1097-0142(19851001)56:7<1704::aid-cncr2820560740>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Pegoraro RJ, Karnan V, Nirmul D, Joubert SM. Estrogen and progesterone receptors in breast cancer among women of different racial groups. Cancer Res. 1986;46:2117–2120. [PubMed] [Google Scholar]
- 3.Elledge RM, Clark GM, Chamness GC, et al. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. JNCI. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 4.Joslyn SA. Hormone receptors in breast cancer: racial differences in distribution and survival. Breast Cancer Res Treat. 2002;73:45–59. doi: 10.1023/a:1015220420400. [DOI] [PubMed] [Google Scholar]
- 5.Morris GJ, Naidu S, Topham AK, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results Database. Cancer. 2007;110:876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 6.Banks E, Canfell K. Recent declines in breast cancer incidence: mounting evidence that reduced use of menopausal hormones is largely responsible. Breast Cancer Res. 2010;12:103. doi: 10.1186/bcr2463. http://breast-cancer-research.com/12/1/103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. New Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Writing Group for the Women's Health Initiative Investigators Risk and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Friedman-Koss D, Crespo CJ, Bellantoni MF, Andersen RE. The relationship of race/ethnicity and social class to hormone replacement therapy: results from the Third National Health and Nutrition Examination Survey 1988–1994. Menopause. 2002;9:264–272. doi: 10.1097/00042192-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 11.Krieger N, Chen JT, Waterman PD. Decline in US breast cancer rates after the women's health initiative: socioeconomic and racial/ethnic differentials. Am J Public Health. 2010;100:S132–139. doi: 10.2105/AJPH.2009.181628. [epub advance access: 10 Feb 2010]. doi:10.2105/AJPH.2009.181628; NIHMS # 171687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Accessed 3 August 2009]; Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) Limited-Use Data (1973–2005), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission. http://seer.cancer.gov/data/index.html.
- 13.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;37(3) [PubMed] [Google Scholar]
- 14.Kim H-J, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60:1005–1014. doi: 10.1111/j.0006-341X.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- 15.Dietel M, Lewis MA, Shapiro S. Hormone replacement therapy: pathobiological aspects of hormone-sensitive cancers in women relevant to epidemiological studies on HRT: a mini-review. Hum Reprod. 2005;20:2052–2060. doi: 10.1093/humrep/dei043. [DOI] [PubMed] [Google Scholar]
- 16.Kunitz S. The health of populations: general theories and particular realities. Oxford University Press; Oxford: 2006. [Google Scholar]
- 17.Krieger N, Davey Smith G. Bodies count & body counts: social epidemiology & embodying inequality. Epidemiol Rev. 2004;26:92–103. doi: 10.1093/epirev/mxh009. [DOI] [PubMed] [Google Scholar]
- 18.Graham H. Smoking prevalence among women in the European community 1950–1990. Soc Sci Med. 1996;43:243–254. doi: 10.1016/0277-9536(95)00369-x. [DOI] [PubMed] [Google Scholar]
- 19.Brandt A. The cigarette century: the rise, fall, and deadly persistence of the product that defined America. Basic Books; New York: 2007. [Google Scholar]
- 20.National Center for Health Statistics . Table 36: death rates for malignant neoplasm of breast among females, by race, Hispanic origin, and age: United States, selected years 1950–2006. National Center for Health Statistics; Hyattsville: 2009. Health, United States 2009; p. 224. [Google Scholar]
- 21.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK, editors. SEER cancer statistics review, 1975–2006. National Cancer Institute; Bethesda: 2010. [Accessed 3 June 2010]. Available at: http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009; see: Table 4.6. Cancer of the breast (Invasive). Age-adjusted US death rates by year, race, and sex. Available at: http://seer.cancer.gov/csr/1975_2006/results_merged/sect_04_breast.pdf. [Google Scholar]
- 22.Krieger N, Chen JT, Ware JH, Kaddour A. Race/ethnicity and breast cancer estrogen receptor status: impact of class, missing data, & modeling assumptions. Cancer Causes Control. 2008;19:1305–1318. doi: 10.1007/s10552-008-9202-1. doi:10.1007/s10552-008-9202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieger N, Emmons K, Williams D. Defining, investigating, and addressing cancer inequities: critical issues. In: Koh H, editor. Toward the elimination of cancer disparities: a clinical and public health perspective. Springer; New York: 2009. pp. 3–28. [Google Scholar]
- 24.Krieger N, editor. Embodying inequality: epidemiologic perspectives. Baywood Publishing Co; Amityville: 2004. [Google Scholar]
- 25.Gravlee CC. How race becomes biology: embodiment of social inequality. Am J Phys Anthropol. 2009;139:47–57. doi: 10.1002/ajpa.20983. [DOI] [PubMed] [Google Scholar]