Abstract
Rosa centifolia and Rosa gruss an teplitz distillation waste biomass was immobilized using sodium alginate for Pb(II) uptake from aqueous solutions under varied experimental conditions. The maximum Pb(II) adsorption occurred at pH 5. Immobilized rose waste biomasses were modified physically and chemically to enhance Pb(II) removal. The Langmuir sorption isotherm and pseudo-second-order kinetic models fitted well to the adsorption data of Pb(II) by immobilized Rosa centifolia and Rosa gruss an teplitz. The adsorbed metal is recovered by treating immobilized biomass with different chemical reagents (H2SO4, HCl and H3PO4) and maximum Pb(II) recovered when treated with sulphuric acid (95.67%). The presence of cometals Na, Ca(II), Al(III), Cr(III), Cr(VI), and Cu(II), reduced Pb(II) adsorption on Rosa centifolia and Rosa gruss an teplitz waste biomass. It can be concluded from the results of the present study that rose waste can be effectively used for the uptake of Pb(II) from aqueous streams.
1. Introduction
Mobilization of the heavy metals in the environment due to industrial activities is of serious concern due to toxicity of these metals in human and other forms of life. Lead (II) is known to be one of the heavy metals most toxic to living organisms and is one of the more wide spread heavy metal contaminants on the environment [1]. Once it enters the environment, it is difficult to recover and it affects the human health [2]. Pb(II) is considered as neurotoxic metal when present above 0.05 mg/L in drinking water [3]. Lead is a metabolic poison and enzyme inhibitor. In young children it can cause mental retardation and semipermanent brain damage. Lead has the ability to form sites for long term release by replacing calcium in the bone. In environmental restoration areas conventional techniques used to eradicate heavy metals from wastewater include precipitation, oxidation/reduction, mechanical filtration, ion exchange resins, membrane separation, adsorption, flocculation, coagulation, and reverse osmosis [4, 5]. However, these processes can be expensive and not fully effective especially when the metal concentration is below 100 mg/L. The other drawbacks of these methods include expensive equipment and monitoring system, high reagents, energy and generation of toxic sludge which requires massive land for dumping. Biosorption has some advantages when compared with conventional methods (1) it is nonpolluting and highly selective; (2) more efficient; (3) easy to operate; (4) cost-effective for treatment of large volumes of wastewaters which contains low concentrations of metals [6]. A biosorbent's immobilization procedure is necessary for the industrial application of biosorption [7]. Everywhere in the world roses are cultivated as important shrubs. These are ornamental plants; they are also used in medicines, perfumes, and for fragrance. From rose, oil is extracted that is used in perfumes and fragrance. Distillation of rose yield three products: Rose oil, rose water, and rose waste biomass [8].
The present study is undertaken (1) to evaluate the usefulness of immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass as a biosorbent for Pb(II) in a single and multicomponent aqueous solutions; (2) to evaluate the effect of different experimental variables like pH, biosorbent dose, initial metal concentration of Pb(II), contact time, column study on sorption process; (3) to investigate the effects of different pretreatments on the sorption capacity (mg/L) of Pb(II); (4) to investigate the metal desorption from loaded waste biomass.
2. Materials and Methods
2.1. Reagents
All chemicals used in these studies were of analytical grade and were purchased from E. Merck Company (Darmstadt, Germany). The reagents used in this study were HCl, H2SO4, NaOH, CaCl2, Lead Acetate, Sodium Alginate, H3PO4, Triton X-100, CH3OH, AlCl3, Ca(OH)2, Al(OH)3, Na2SO4, K2Cr2O7, and Pb(II) standard solution 1000 mg/L. The stock solution of lead was prepared by dissolving 1.83 g of Lead acetate (CH3COOPb) in 100 mL of deionized distilled water (DDW) and diluted up to 1000 mL. Then solutions of desired concentrations were prepared from the stock solution.
2.2. Biomass
In the present study the rose distillation sludge was selected as biosorbent. Rosa centifolia and Rosa gruss an teplitz waste biomass used in this work was harvested from Rose Laboratory, Institute of Horticultural sciences, University of Agriculture, Faisalabad, Pakistan. The obtained biomass was washed with distilled water to remove impurities and particulate matter. The obtained biomass was first sun dried and then oven dried at 60°C for 24 hour to obtain biomass in completely dried form. Standard sampling techniques were used to ensure the collection of homogenous sample. The dried waste biomass was then pulverized and finally sieved (0.25 mm). The biomass was immobilized by mixing 1 g of dried biomass and 2 g Na alginate in 100 ml of deionized distilled water. The alginate-biomass mixture was extruded through a 100 mL burette into 0.15 M CaCl2 solution for bead formation. The spherical beads (3.5 mm) were preserved in 50 mM CaCl2 until further use.
2.3. Pretreatment of Biomass
The immobilized Rosa centifolia and Rosa gruss an teplitz biomass was physically modified by heating (0.1 g of beads in an electric oven at 60°C for 30 minutes) and boiling (0.1 g of beads in 100 ml of deionized distilled water for 30 minutes). One gram of immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass pretreated using 1000 mL of 0.1 N H2SO4, H3PO4, Ca(OH)2, Al(OH)3, and CH3OH for 24 hours. The surface of immobilized biomass (1 g) was mobilized using 1000 ml of 1% Triton X-100 for 24 hours. The immobilized biomass weight was taken on dry weight basis during the present study (0.1 g of dried immobilized biomass was equal to 1 g of fresh immobilized biomass).
2.4. Biosorption Studies
The effect of pH (1, 2, 3, 4, and 5), biosorbent dose (0.05, 0.1, 0.2, 0.3, and 0.4 g/100 mL), initial metal concentration (25, 50, 100, 200, 400, and 800 mg/L), contact time (5, 10, 15, 30, 60, 120, 240, 360, 720, and 1440 minutes), and Co-metals ions (Na, Ca(II), Al(III), Cr(III), Cr(VI), Cu(II)) on the sorption of Pb(II) were evaluated during the present study. The pH of aqueous medium was adjusted using 0.1 M HCl and 0.1 M NaOH were used. In the end of each experiment, solution was separated from the waste biomass by filtration. The controls were accompanied each experiment. The concentration of Lead in the solution before and after the experiment was determined by using Flame Atomic Absorption Spectrophotometer (FAAS), using a Perkin-Elmer AAnalyst 300 (FAAS) equipped with air-acetylene burner. The selected analytical wavelength for Lead was 232 nm. The instrument was periodically checked by known standards to ensure the accuracy. For each sample three readings were taken and mean of these three was computed along with standard deviations for each sample. The amount of metal biosorbed on each waste biomass was assumed to the difference between initial metal concentration and that present in the solution. Uptake was calculated by concentration difference method. The uptake of Pb(II) was calculated by the simple concentration method. The metal uptake (qe) and % age removal were calculated from the mass balance and % age removal equations as follows:
| (1) |
where V is the volume of solution in mg/L and w is the mass of sorbent, Ci is the initial concentration (mg/L), and Ce is the metal concentration at the various time intervals and qe is the metal uptake in (mg/g).
The extent of sorption in percentage is found from the relation
| (2) |
2.5. Statistical Analysis
The data given in this study represents the mean of three independent experiments. All results are evaluated by mean ± SD values. The correlation coefficient (R2) values of the linear form of Langmuir isotherm, Freundlich isotherm, pseudo-first-order and pseudo-second-order models were also determined using statistical functions of Microsoft Excel (version Office XP, Microsoft Cooperation, USA).
3. Results and Discussions
3.1. Effect of pH
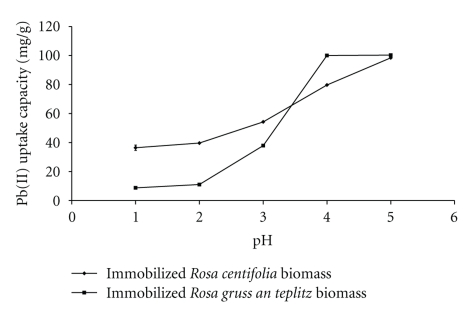
One of the most important factors in the biosorption studies is the degree of acidity of the medium. Aqueous solution pH is a critical parameter as it strongly affects metal biosorption, surface charge of the adsorbent, degree of ionization, and speciation of adsorbate species. It affects the solution chemistry of metals, the activity of functional groups in the biomass, and competition of the metallic ions [6]. Maximum lead removal was observed at pH 5 (Figure 1). When pH was further increased, the soluble lead became precipitated. With an increase in pH, the functional groups on the cell wall with negative charge increase due to deprotonation of the metal binding sites, which promotes the metal uptake [3]. Nasir et al., [8] also observed maximum lead removal at pH 5 with untreated and treated waste biosorbent. It was reported that at highly acidic pH (<3) lead (II) ions compete with H+ on the binding sites of cells and adsorption is lowered. When the solution pH increased beyond 5, the insoluble precipitates formed making true sorption studies impossible [9].
Figure 1.
Effect of pH on Pb(II) uptake by immobilized rose waste biomass.
3.2. Effect of Biosorbent Dose
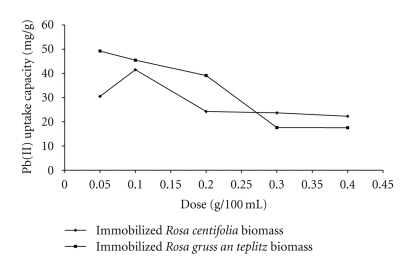
The results show that maximum biosorption by immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass occurred at 0.05 g/100 ml for Pb(II) solution (Figure 2). This shows that the maximum adsorptions occur at minimum dose and hence the amount of ions bound to the adsorbent and amount of free ions remained constant. After this dose, the uptake capacity (mg/g) of biosorbent was gradually decreased with increase in dose. Amount of biosorbent added to the solution determines the number of binding sites available for adsorption. The results can be attributed to some kind of hindrance as due to partial aggregation/agglomeration of sorbet particles at higher concentration (Figure 2). Therefore, a more economical removal of a given amount of metal ions can be carried out using small batches of sorbent rather than in a single batch [10, 11].
Figure 2.
Effect of pH on Pb(II) uptake by immobilized rose waste biomass.
3.3. Effect of Initial Metal Concentration
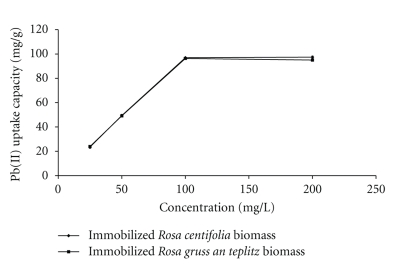
Removal of Pb(II) by immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass at different metal concentrations (25, 50, 100, 200, 400, and 800 mg/L), at constant pH 5 is shown in the Figure 3. The obtained results indicated that Pb(II) sorption capacity of immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass increased up till 200 mg/L afterwards a decrease was observed. This sorption characteristic has indicated that surface saturation is dependent on the initial metal ion concentrations. At low concentration, the ratio of sorptive surface area to the total metal ions available is high; adsorption sites have taken the available metal more quickly. The difference between the bulk and surface metal ions concentration is one of the driving forces to overcome the resistance to sorption process, in the absence of mass transfer resistances, surface and bulk concentration are identical, thus, enhancing the sorption process. However, at higher concentrations, metals need to diffuse to the biomass surface by intraparticle; diffusion and greatly hydrolyzed ions will diffuse at a slower rate [12]. There are several equations of isotherm which have been used for equilibrium modeling for the systems of biosorption [2]. For this study, two frequently used equations Langmuir and Freundlich isotherm models were selected. In Langmuir model the maximum adsorption capacity qmax (mg/g) and other parameters were determined from
| (3) |
where qe is metal sorption capacity (mg/g), Ce the equilibrium concentration of metal ions solution, KL is the Langmuir adsorption constant.
Figure 3.
Effect of initial metal concentration on Pb(II) uptake by immobilized rose waste biomass.
The adsorption on heterogeneous surface of biomass can be determined by the log form of Freundlich isotherm in
| (4) |
where k and n are Freundlich constants.
Table 1 shows the correspondent constants along with the coefficients of correlation (R2) associated to each linearized model. The results indicated that the model better fitted to the data was Langmuir model due to higher value of its correlation coefficient. The well fitting of experimental data to Langmuir model clearly demonstrated that Pb(II) sorption onto immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass was a surface phenomenon.
Table 1.
Comparison between Langmuir and Freundlich adsorption isotherm parameters for Pb(II) sorption by immobilized rose waste biomass.
| Biosorbent | Langmuir isotherm parameters | Experimental value | Freundlich isotherm parameters | |||||
|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (l/mg) | R2 | qmax (mg/g) | qmax (mg/g) | K (mg/g) | 1/n | R2 | |
| Rosacentifolia | 101.01 | 0.2964 | 0.9979 | 97.4 | 117.76 | 1.508 | 0.2809 | 0.5515 |
| Rosa gruss an teplitz | 102.04 | 0.1884 | 0.9932 | 97.2 | 365.51 | 1.9484 | 0.3065 | 0.3965 |
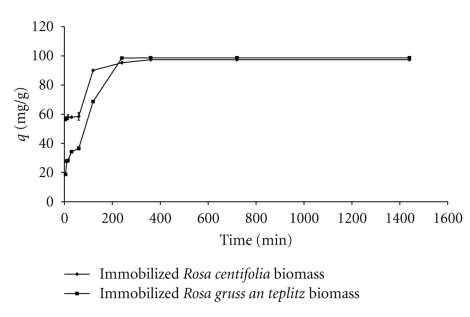
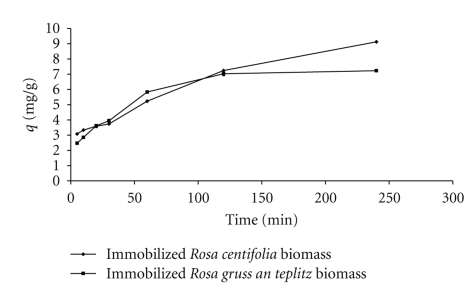
3.4. Effect of Contact Time
The equilibrium time is one of the important parameters for an economical wastewater treatment system [13–18]. The effect of the contact time on Pb(II) biosorption immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass was studied by varying time interval in range of 5–1440 minutes at constant values of pH (5), biosorbent dose (0.1 g/100 mL), and initial metal concentration (100 mg/L) at 30°C (Figure 4). It was observed that an equilibrium time of 240 minutes was necessary to reach the equilibrium for Pb(II) biosorption. A further increase in time did not show an increase in biosorption. It is well known that rate of metal uptake is influenced by factors effecting mass transfer from bulk solution to binding sites [6]. To understand the adsorption mechanism of Pb(II) on to immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass, modeling of data was performed using Lagergren pseudo-first-order and pseudo-second-order kinetic models. The linearized form of pseudo-first-order equation (5) is given as
| (5) |
where qe is the mass of metal adsorbed at equilibrium (mg/g), qt is the mass of metal at time t (min.), k1,ads is the first-order reaction rate of adsorption (per min.).
Figure 4.
Effect of contact time on Pb(II) uptake by immobilized rose waste biomass.
The pseudo second order is expressed in
| (6) |
where k2 is the rate constant of pseudo-second-order biosorption model (g/mg/min).
A comparison (Table 2) between kinetic models suggested that the coefficient of correlation (R2) for the pseudo-second-order kinetic model is much higher in comparison to pseudo-first-order model. The value of experimental qe (mg/g) and qmax (mg/g) is in good agreement for pseudo-second-order kinetic model. So, it is suggested that the model fitted to data was pseudo-second-order kinetic model.
Table 2.
A comparison between Lagergren pseudo-first-order and pseudo-second-order kinetic models for Pb(II) uptake by immobilized rose waste biomass.
| Biosorbent | Pseudo-first-order kinetic model | Experimental value | Pseudo-second-order kinetic model | ||||
|---|---|---|---|---|---|---|---|
| qe(mg/g) | K1,ads (min−1) | R2 | qe (mg/g) | qe (mg/g) | K2,ads (g mg−1 min−1) | R2 | |
| Rosa centifolia | 51.59 | 0.0133 | 0.9455 | 97.399 | 101.01 | 5.99×10−4 | 0.9912 |
| Rosa gruss an teplitz | 147.8 | 1.059 | 0.8786 | 98.67 | 113.64 | 0.774 x10−4 | 0.9585 |
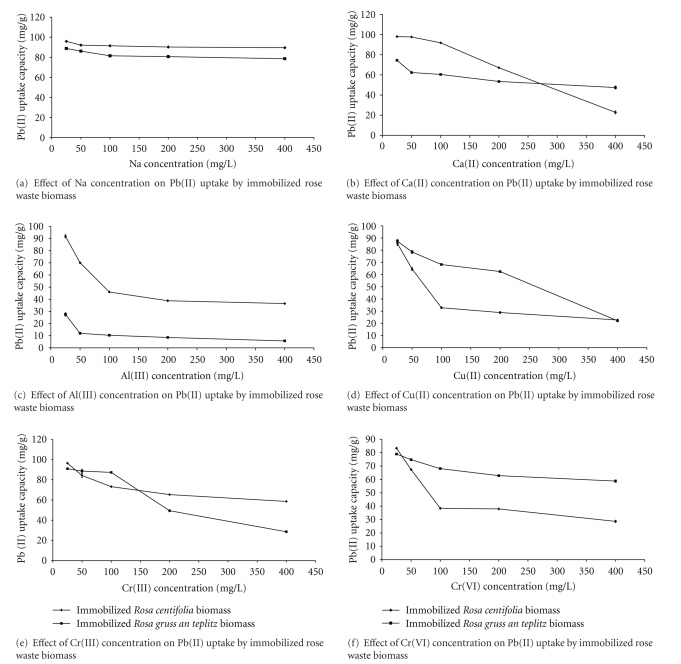
3.5. Effect of Co-Metals
In order to investigate the effect of other metal ions, present in the aqueous solution, on the uptake capacity (mg/g) of Pb(II) immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass, a study was conducted at a constant values of pH 5, biosorbent dose (0.1 g/100 mL), and initial metal concentration (100 mg/L) at 30°C (Figures 5(a)–5(f)). Among all the metals studied, Pb(II) has highest electronegativity value (2.33), so, having highest affinity for binding sites. The order of electronegativity for other metals is given as Na(I)0.93 < Ca(II)1.00 < Al(III)1.61 < Cr(II)1.66 < Cr(VI)1.66 < Cu(II)1.9 < Pb(II)2.33. When we consider atomic number as a major factor affecting uptake capacity of Pb(II) by immobilized waste biomass, then, the order of atomic numbers for the co-metals studied is Pb(II)82 > Cu(II)29 > Cr(III)24 > Cr(VI)24 > Ca(II)20 > Al(III)13 > Na(I)11. Because of highest atomic number from all other studied metals, Pb(II) can easily replace other metal ions from the binding sites. The higher value of ionic radii for Pb(II) 1.32, also favored Pb(II) sorption as compared to other metals. Pb(II) also has highest Standard Potential value (−0.126) E° (Volts) after Cu(II), so it can easily replace any metal from biomass surface. Among all the co-metals studied in this experiment, only Al(III) has strongly affected the sorption capacity of Pb(II) by immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass due to it is higher ionic charge than Pb(II). After Al(III), second competing metal ion with Pb(II), was Cu(II). This could be due to higher Standard potential values for Cu(II) (0.34 E° Volts), as compared to Pb(II) (−0.126 E° Volts), which enabled Cu(II) to replace Pb(II) from the binding sites. From the studies of the competitive adsorption of Cu(II) and Pb(II), we can come to a conclusion that because both metals have same ionic charge, mechanism of the two metal ions is in homology nearly. The tendency for adsorbed Pb(II) was higher than that for Cu(II) at the initial process [19]. After Cu(II), the metal who competed most with Pb(II) for binding sites, was Cr(VI). This could be probably due to higher ionic charge. Other metal ions like Na(I), Ca(II), and Cr(III) also effected the sorption capacity of Pb(II) by immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass to some extent. The results showed that there was a decrease in Pb(II) uptake as the concentration of co-metal ion increased. In the presence of coions in solution, chemical interactions between the ions themselves as well as with the biomass, take place resulting in site competition. Many of the functional groups present on the cell wall and the membrane are nonspecific and different cations compete for the binding sites. It has been reported that metal removal is increased as the ionic radii of metal cations affect the ion exchange and adsorption process. The differences in the sorption affinities may also be attributed to differences in the electrode potentials of the various ions. The greater the electrode potential, the greater is the affinity for biomass [13]. The process of metal removal is inhibited in the presence of other ions. The presence of a multiplicity of metals leads to interactive effect. These effects can be extremely complex and three types of responses may be expected: (i) the effects of mixture is greater than that of the individual effects of ions in the mixture (synergism); (ii) the effects of mixture is less than that of the individual effects of ions in the mixture (antagonism); (iii) no effect of mixture (no interaction). The actual mechanisms of heavy metal adsorption especially multicomponents are not well understood and need further detailed studies.
Figure 5.
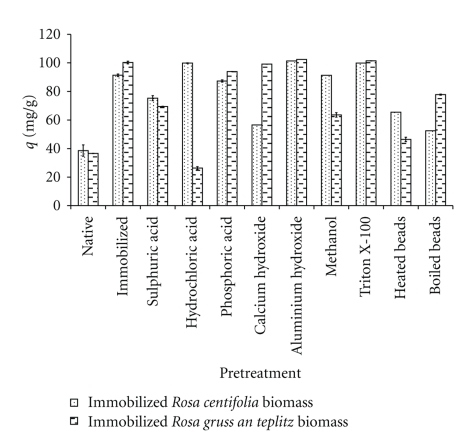
3.6. Effect of Pretreatment
An increase in biosorption of lead (II) ions as a result of pretreatment could be due to an exposure of active metal binding sites embedded in the cell wall or chemical modifications of the cell wall components. The effect of pretreatment on uptake capacity of immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass is shown in Figure 6. The metal sorption capacity (mg/g) of physically treated Rosa centifolia was Immobilized (91.36) > Heated immobilized (65.43) > Boiled immobilized (52.47) > Native (38.61). The metal sorption capacity (mg/g) of physically treated immobilized Rosa gruss an teplitz waste biomass was Boiled (77.78) > Heated (46.43) > Native (36.62). Boiling removes mineral matter from biomass and increase number of vacant biosorbents sites. Heating biomass results in decomposition of organic matter and increases the metal uptake by increasing newly vacant biosorbent sites [14]. After alkali pretreatment biosorption capacity (mg/g) of immobilized Rosa centifolia waste biomass was Al(OH)3 (101.32) > Ca(OH)2 (56.57). The metal sorption capacity (mg/g) of alkali pretreated immobilized Rosa gruss an teplitz waste biomass was Al(OH)3 (99.3) > Ca(OH)2 (99.04). Autolytic enzymes may be destroyed by alkali treatment which cause putrefaction of biomass and remove proteins and lipids that mask reactive sites [15]. After acid pretreatment, biosorption capacity (mg/g) of immobilized Rosa centifolia waste biomass was: HCl (99.834) > H3PO4 (87.36) > H2SO4 (75.27). After acid pretreatment, the biosorption capacity (mg/g) of immobilized Rosa gruss an teplitz waste biomass was H3PO4 (91.71) > H2SO4 (69.5) > (HCl) (26.38). After acid pretreatment a negative charge occurs on the surface of biomass due to ionization of organic and inorganic groups. Increased charge on biomass surface was responsible for increase in Pb(II) uptake capacity of biomass. Methanol and Triton X-100 pretreatment of both biosorbents resulted in increase in Pb(II) uptake capacity. Triton X-100 is nonionic surfactant. The sorption of heavy metal to the biomass can be increased in the presence of surfactants because they reduce the surface tension as well as increasing wetting power, allowing easier spreading and lowering the interfacial tension between two substances [15]. Pretreatment with methanol results in the methylation of amino groups present on the surface of waste biomass. Drake et al. [16] also observed that the treatment of biomass with methanol result in esterification of carboxylic acids present on the cell wall of biosorbents [17].
Figure 6.
Effect of biosorbent pretreatment on Pb(II) uptake by immobilized rose waste biomass.
3.7. Column Study
For practical applications it is necessary to determine the maximum Pb(II) uptake in column setup. Column study was conducted at pH 5, initial metal concentration 100 mg/L, biosorbent dose 0.5 g, for a time range of 5, 10, 20, 30, 60, 120, and 240 minutes. The equilibrium time for column setup was 240 minutes in comparison to 360 minutes in case of batch setup (Figure 7). Modelling of the data was done using pseudo-first-order kinetic model and pseudo-second-order model. A comparison between kinetic models is given in Table 3, which suggested that the coefficient of correlation (R2) for the pseudo-second-order kinetic model is much higher as comparison to pseudo-first-order kinetic model. The value of experimental qe (mg/g) and qmax (mg/g) is in good agreement for pseudo-second-order kinetic model. Column setup was found to have significantly low adsorption capacity in comparison to batch set up [18].
Figure 7.
Effect of contact time on Pb(II) uptake by immobilized rose waste biomass in column setup.
Table 3.
A comparisons between Lagergren pseudo-first-order and pseudo-second-order kinetic models for Pb(II) uptake by immobilized rose waste biomass in column setup.
| Biosorbent | Pseudo-first-order kinetic model | Experimental value | Pseudo-second-order kinetic model | ||||
|---|---|---|---|---|---|---|---|
| qe(mg/g) | K1,ads (min−1) | R2 | qe (mg/g) | qe (mg/g) | K2,ads (g mg−1 min−1) | R2 | |
| Rosa centifolia | 6.74 | 0.0103 | 0.9806 | 9.122 | 9.98 | 2.93 | 0.9631 |
| Rosa gruss an teplitz | 1.98 | 0.0115 | 0.4495 | 7.028 | 6.83 | 0.09862 × 10−4 | 0.9607 |
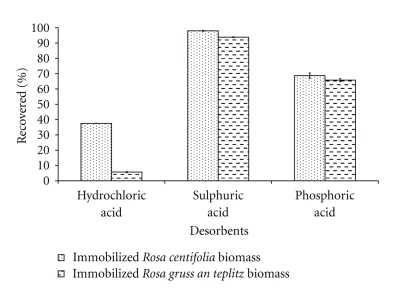
3.8. Desorption Experiments
Disposal of exhausted adsorbent loaded with heavy metal ions creates another environmental problem as it is hazardous material which pollute environment. This problem may be overcome to some extent by using one of the elimination (e.g., elution, incineration, and pyrolysis) methods. The elution of heavy metal is the most common elimination method, allowing both recovery of solution of heavy metal ions at higher concentration for inertisation and recycling of the adsorbent for subsequent uses [19]. The desorption of loaded immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass was evaluated using 0.1 N HCl, H2SO4, and H3PO4, used as desorbing agents (Figure 8). The percentage recovery of Pb(II) from loaded pretreated with 0.1 N acids, waste biomass is in order: H2SO4> H3PO4> HCl. The high percentage recovery of Pb(II) allows the reuse of biomass and is an important feature for its possible use in continuous system in industrial process and in analytical procedures for preconcentration of trace elements.
Figure 8.
Desorption of Pb(II) by immobilized rose waste biomass.
3.9. Fourier Transform Infrared Spectroscopic (FTIR) Analysis
In order to find out functional groups responsible for Pb(II) biosorption, FTIR analysis of the biomass was carried out before and after metal uptake. FTIR spectra shows the involvement of amino (–NH), carboxylate anions (–COO−), hydroxy (–OH), and others (–C–N), (–C–O), (–C–H), (–C=O) which present different affinities towards metallic ions [27]. The broadband after 3500 cm−1 indicates O–H stretching and the lower intensity band near 2900 cm−1 represents stretching of the O–H groups bound to methyl and methylene radicals; these groups are present on the lignin structure. A broad, intense –OH stretching absorption band from 3300 to 2500 cm−1 present in FTIR spectrum of biomasses indicated the carboxylic functional group present on biomass cell surface. Weaker –CH stretch bands are superimposed onto the side of the broad –OH band at 3000–2800 cm−1. The peaks located at 1800–1600 cm−1 are characteristics of carbonyl group stretching from aldehydes and ketones. Aromatic rings stretching bands associated with C–C can be seen between 1600 to 1500 cm−1. The bands present near 1450 cm−1 indicated that the –NH was also involved in metal biosorption. The absorbance of the peaks in the metal loaded sample was substantially lower than those in the raw sample. This indicated that bond stretching occurred to a lesser degree due to the exchange of hydrogen ions with Pb(II), and subsequently peak absorbance was attenuated.
3.10. Pb(II) Uptake Capacity Comparison
A comparison between Pb(II) uptake capacity of immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass with some previously used biosorbents is tabulated in Table 4 [20–26]. From Table 4 it can be concluded that immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass has more sorption capacity in comparison to most of the earlier reported biosorbents.
Table 4.
A comparison between immobilized rose waste biomass and previously used biosorbent for the uptake of Pb(II).
| Biosorbent | Uptake capacity (mg/g) | Reference |
|---|---|---|
| Immobilized Rosa centifolia | 97.399 | Present study |
| Immobilized Rosa gruss an teplitz | 98.67 | |
| Ceratophyllum demersum | 44.80 | [20] |
| Myriophyllum spicatum | 46.49 | [21] |
| Aspergillus flavus | 10.82 | [22] |
| Waste chinese herb Pang Da Hai | 27.10 | [23] |
| Bacillus sp. | 92.27 | [24] |
| Chlamydomonas reinhardtii | 96.30 | [25] |
| Brown seaweed Cystoseira baccata. | 186 | [26] |
4. Conclusions
Following are the main conclusions that can be withdrawn from the present study.
The Pb(II) uptake by immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass was found highly dependent on physiochemical parameters such as pH, dosage, chemical pretreatment, initial metal concentration, co-metal ion concentration, and contact time as well as on batch or continuous setup.
Alkali pretreatment of immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass using Al(OH)3 was found more effective than chemical and physical pretreatments to increase uptake capacity of biomass.
Langmuir adsorption isotherm and pseudo second-order models were best fitted to the experimental results.
Pb(II) was effectively desorbed from immobilized Rosa centifolia and Rosa gruss an teplitz waste biomass using acids. The effectiveness of various acids towards Pb(II) desorption was found as follows: H2SO4> H3PO4> HCl.
FTIR analysis of rose waste biomass indicates the involvement of carbonyl, carboxyl, hydroxyl, aromatic, and amino functional groups in Pb(II) uptake process.
References
- 1.Dönmez G, Aksu Z. Bioaccumulation of copper(II) and nickel(II) by the non-adapted and adapted growing CANDIDA SP. Water Research. 2001;35(6):1425–1434. doi: 10.1016/s0043-1354(00)00394-8. [DOI] [PubMed] [Google Scholar]
- 2.Nadeem R, Ansari TM, Khalid AM. Fourier Transform Infrared Spectroscopic characterization and optimization of Pb(II) biosorption by fish (Labeo rohita) scales. Journal of Hazardous Materials. 2008;156(1–3):64–73. doi: 10.1016/j.jhazmat.2007.11.124. [DOI] [PubMed] [Google Scholar]
- 3.Sag Y, Ozer D, Kutsal T. A comparative study of the biosorption of lead(II) ions to Z. ramigera and R. arrhizus. Process Biochemistry. 1995;30(2):169–174. [Google Scholar]
- 4.Mehta SK, Gaur JP. Use of algae for removing heavy metal ions from wastewater: progress and prospects. Critical Reviews in Biotechnology. 2005;25(3):113–152. doi: 10.1080/07388550500248571. [DOI] [PubMed] [Google Scholar]
- 5.Basha S, Murthy ZP. Seaweeds for engineering metal biosorption: are view. In: Mason LG, editor. Focus on Hazardous Materials Research. Hauppauge, NY, USA: Nova Science; 2007. pp. 165–209. [Google Scholar]
- 6.Hanif MA, Nadeem R, Bhatti HN, Ahmad NR, Ansari TM. Ni(II) biosorption by Cassia fistula (Golden Shower) biomass. Journal of Hazardous Materials. 2007;139(2):345–355. doi: 10.1016/j.jhazmat.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hakawati MS, Banks CJ. Copper removal by polymer immobilised Rhizopus oryzae. Water Science and Technology. 2000;42(7-8):345–352. [Google Scholar]
- 8.Nasir MH, Nadeem R, Akhtar K, Hanif MA, Khalid AM. Efficacy of modified distillation sludge of rose (Rosa centifolia) petals for lead(II) and zinc(II) removal from aqueous solutions. Journal of Hazardous Materials. 2007;147(3):1006–1014. doi: 10.1016/j.jhazmat.2007.01.131. [DOI] [PubMed] [Google Scholar]
- 9.Ho Y-S. Effect of pH on lead removal from water using tree fern as the sorbent. Bioresource Technology. 2005;96(11):1292–1296. doi: 10.1016/j.biortech.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Montanher SF, Oliveira EA, Rollemberg MC. Removal of metal ions from aqueous solutions by sorption onto rice bran. Journal of Hazardous Materials. 2005;117(2-3):207–211. doi: 10.1016/j.jhazmat.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Quek SY, Al-Duri B, Wase DAJ, Forster CF. Coir as a biosorbent of copper and lead. Process Safety and Environmental Protection. 1998;76(1):50–54. [Google Scholar]
- 12.Saeed A, Iqbal M, Akhtar MW. Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk) Journal of Hazardous Materials. 2005;117(1):65–73. doi: 10.1016/j.jhazmat.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Mattuschka B, Straube G. Biosorption of metals by a waste biomass. Journal of Chemical Technology and Biotechnology. 1993;58(1):57–63. [Google Scholar]
- 14.Zubair A, Bhatti HN, Hanif MA, Shafqat F. Kinetic and equilibrium modeling for Cr(III) and Cr(VI) removal from aqueous solutions by Citrus reticulata waste biomass. Water, Air, and Soil Pollution. 2008;191(1–4):305–318. [Google Scholar]
- 15.Muraleedharan TR, Venkobachar C. Mechanism of cobalt biosorption. Biotechnology and Bioengineering. 1990;33:823–831. [Google Scholar]
- 16.Drake LR, Lin S, Rayson GD, Jackson PJ. Chemical modification and metal binding studies of Datura innoxia. Environmental Science and Technology. 1996;30(1):110–114. [Google Scholar]
- 17.Gardea-Torresday JL, Cano-Aguilera I, Tiemann JK, Webb R, Gutierrez-Corona F. Copper binding by inactivated cells of Mucor rouxii. In: Proceedings of the 10th Annual Conference on Hazardous Waste Research; May 1995; Manhattan, Kan, USA. pp. 33–40. [Google Scholar]
- 18.Ho Y-S. Effect of pH on lead removal from water using tree fern as the sorbent. Bioresource Technology. 2005;96(11):1292–1296. doi: 10.1016/j.biortech.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Han R, Zhang J, Zou W, Xiao H, Shi J, Liu H. Biosorption of copper(II) and lead(II) from aqueous solution by chaff in a fixed-bed column. Journal of Hazardous Materials. 2006;133(1–3):262–268. doi: 10.1016/j.jhazmat.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Keskinkan O, Goksu MZL, Basibuyuk M, Forster CF. Heavy metal adsorption properties of a submerged aquatic plant (Ceratophyllum demersum) Bioresource Technology. 2004;92(2):197–200. doi: 10.1016/j.biortech.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Keskinkan O, Goksu MZL, Yuceer A, Basibuyuk M, Forster CF. Heavy metal adsorption characteristics of a submerged aquatic plant (Myriophyllum spicatum) Process Biochemistry. 2003;39(2):179–183. [Google Scholar]
- 22.Akar T, Tunali S. Biosorption characteristics of Aspergillus flavus biomass for removal of Pb(II) and Cu(II) ions from an aqueous solution. Bioresource Technology. 2006;97(15):1780–1787. doi: 10.1016/j.biortech.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Chang X, Guo Y, Meng S. Biosorption and preconcentration of lead and cadmium on waste Chinese herb Pang Da Hai. Journal of Hazardous Materials. 2006;135(1–3):389–394. doi: 10.1016/j.jhazmat.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 24.Tunali S, Çabuk A, Akar T. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chemical Engineering Journal. 2006;115(3):203–211. [Google Scholar]
- 25.Tüzün I, Bayramoğlu G, Yalçin E, Başaran G, Çelik G, Arica MY. Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. Journal of Environmental Management. 2005;77(2):85–92. doi: 10.1016/j.jenvman.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Lodeiro P, Barriada JL, Herrero R, Sastre de Vicente ME. The marine macroalga Cystoseira baccata as biosorbent for cadmium(II) and lead(II) removal: kinetic and equilibrium studies. Environmental Pollution. 2006;142(2):264–273. doi: 10.1016/j.envpol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Selatnia A, Boukazoula A, Kechid N, Bakhti MZ, Chergui A, Kerchich Y. Biosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Biochemical Engineering Journal. 2004;19(2):127–135. [Google Scholar]