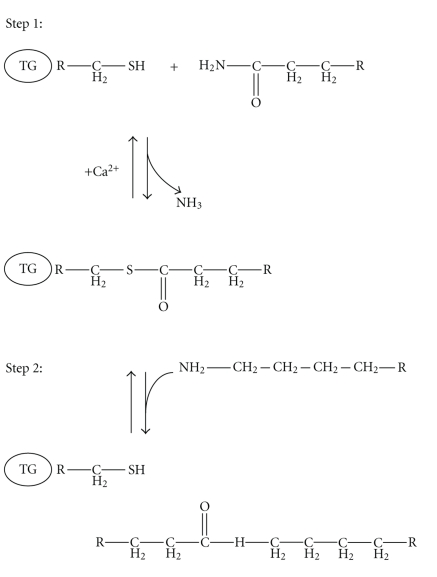
Figure 2.
Schematic representation of a two-step transglutaminase reaction. Step 1: In the presence of Ca2+, the active-site cysteine residue reacts with the γ-carboxamide group of an incoming glutaminyl residue of a protein/peptide substrate to yield a thioacyl-enzyme intermediate and ammonia. Step 2: The thioacyl-enzyme intermediate reacts with a nucleophilic primary amine substrate, resulting in the covalent attachment of the amine-containing donor to the substrate glutaminyl acceptor and regeneration of the cysteinyl residue at the active site. If the primary amine is donated by the ε-amino group of a lysyl residue in a protein/polypeptide, an Nε-(γ-L-glutamyl)-L-lysine (GGEL) isopeptide bond is formed.