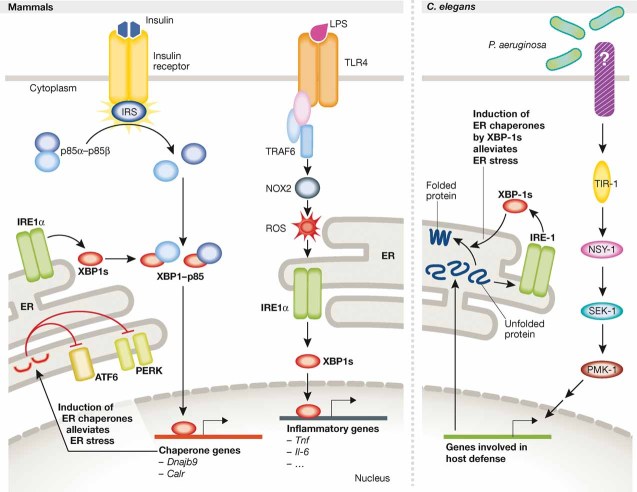
Figure 1. IRE1/XBP1 links ER homeostasis to metabolism and innate immunity.
Upon insulin stimulation, heterodimers of p85α–p85β, the regulatory subunits of PI3K, dissociate so that p85 can interact with XBP1, leading to its nuclear translocation (left panel, left part). XBP1 in the nucleus induces expression of ER chaperones thereby alleviating ER stress, preventing chronic UPR activation and improving insulin sensitivity. In macrophages, TLR2/4 specifically activates IRE1α-dependent production of XBP1s to induce transcription of inflammatory response genes, including Il6 and Tnf, by binding to their promoter regions (left panel, right part). Upon TLR activation, the adaptor protein TRAF6 mediates the NOX-2-dependent production of reactive oxygen species (ROS), which are required for the activation of IRE1α. When Caenorhabditis elegans larvae are infected by Pseudomonas aeruginosa, activation of the PMK-1-mediated immune response induces expression of a large set of genes that increases the protein-folding load on the ER (right panel). The accumulation of unfolded proteins activates IRE-1, thus leading to the production of XBP1s, which enhances productive protein folding by chaperone gene induction and protects the larvae from immune response-induced ER stress.