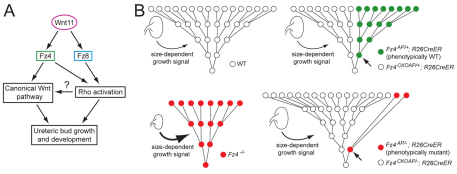
Fig. 8.
Models of Wnt11 signaling and of cell proliferation and competition in the developing mouse kidney. (A) Wnt11 signals via Fz4 through both the canonical and noncanonical Wnt pathways, but via Fz8 only through the noncanonical pathway. (B) Inverted pyramids show several rounds of cell division during ureteric bud development. Time proceeds from bottom to top in each panel, and the relative rates of cell proliferation are reflected in the time between cell divisions. In WT kidneys (upper left) and in genetically mosaic kidneys (upper right), in which AP+ cells (green) are phenotypically WT, cell proliferation is rapid and only a relatively weak kidney size-dependent growth-promoting signal further augments proliferation (thin curved arrow). In Fz4−/− or Fz4−/−;Fz8−/− kidneys (lower left), reduced Wnt signaling leads to reduced cell proliferation, but this is partially compensated by strong activation of a kidney size-dependent growth-promoting signal (thick curved arrow). In genetically mosaic kidneys (lower right) in which marked cells (red) are Fz4−/− or Fz4−/−;Fz8−/−, the higher proliferation rate of phenotypically WT Fz4+/− or Fz4+/−;Fz8−/− cells eventually leads to a predominantly WT ureteric bud. In this last situation, the model predicts that the kidney size-dependent growth-promoting signal would be only modestly activated (medium arrow), leaving the Fz4−/− or Fz4−/−;Fz8−/− cells to proliferate at a greatly reduced rate. In each of the two right-hand diagrams, the small arrow points to the founder cell in the clone of Cre-recombined cells.