Figure 4.
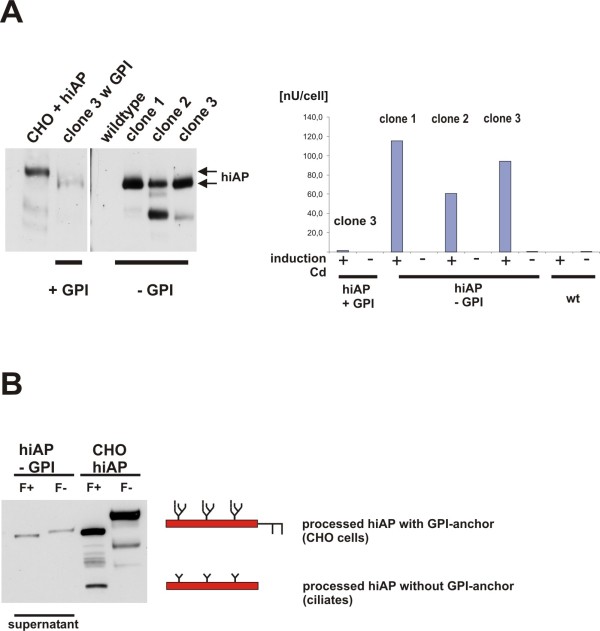
Secretion of hiAP that lacks the GPI anchor signal. A: Expression and secretion of functional recombinant hiAP. We analyzed the supernatant of three clones that were induced with cadmium. The clones carried the expression cassette that encodes hiAP without the GPI anchor signal. As control we used the clone shown in Figure 2 (clone 3 +GPI), the wild type and an extract from CHO cells that express hiAP. Clone 1, 2 and 3 show a positive signal in the Western blot analysis, the additional bands are due to protein degradation (clone 2 and 3). As expected almost no hiAP expression signal was observed in the supernatant of cells expressing hiAP +GPI (clone 3 +GPI). The enzyme activity assay (right side of the figure) confirms the data of the Western blot analysis. Clone 2 and 3 are positive, but the highest activity was found in the supernatant of clone 1. No or only few enzyme activity was found in supernatants of the wild type and in the supernatant of the clone that expresses full-length hiAP. The activity is given in nU/cell (measured activity per cell) to allow a direct comparison between the supernatant of the different clones. B: N-glycosylation of secreted hiAP. We treated the supernatant of one clone with PNGase F and analyzed the samples by SDS-PAGE and Western blot. As a control we used the extract from CHO cells that express hiAP. We found a significant band shift, suggesting that also truncated hiAP becomes glycosylated during the passage through the ER and Golgi compartments like the full-length hiAP protein. The band shift in the ciliate samples is less significant due to the smaller oligo-mannose N-glycan structure. The scheme illustrates the different post-translational modification in ciliates compared to the CHO cells.