Figure 1.
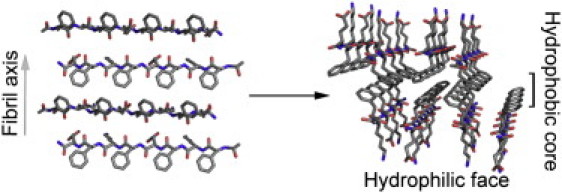
Proposed packing mode of the cofacial bilayer for (FKFE)2. (Left) (FKFE)2β-strands in an antiparallel registry forming a β-sheet; the figure is arranged to illustrate the direction of the fibril axis with the hydrophobic amino-acid side chains extending out of the page and the hydrophilic amino-acid side chains descending into the page. (Right) The cofacial assembly of two β-sheets to form the proposed bilayer architecture that comprises the basic fibrillar unit. This cofacial assembly is facilitated by burial of the hydrophobic side chains in a hydrophobic core, leaving the hydrophilic side chains exposed at the fibril surface. This arrangement accounts for the high water solubility of these peptide fibrils. (Colors: Red corresponds to oxygen, blue to nitrogen, and gray to carbon.)
