Figure 2.
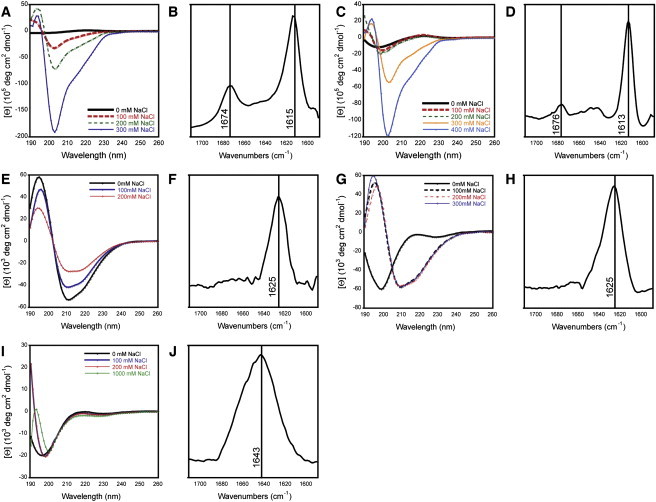
Spectral characterization of self-assembled structures formed by synthetic cationic peptides. (A, C, E, G, and I) CD spectra of cationic peptides in unbuffered water. Peptides were dissolved in unbuffered water at a peptide concentration of 0.9 mM, in the presence of increasing concentrations of sodium chloride. Spectra are labeled as follows: (A) K2(FKFE)2; (C) K4(FKFE)2; (E) K2(ChaKChaE)2; (G) K4(ChaKChaE)2; (I) K2(AKAE)2. (B, D, F, H, and J) FTIR spectra of cationic peptides in unbuffered water. The TFA counterions that are present with each peptide as a function of HPLC purification were exchanged by lyophilization from HCl: peptides were then lyophilized from D2O. Peptides were then dissolved in D2O (1.5 mM) containing the appropriate amount of NaCl necessary to induce the formation of β-sheet assemblies. IR spectra are labeled as follows: (B) K2(FKFE)2 [NaCl = 100 mM]; (D) K4(FKFE)2 [NaCl = 300 mM]; (F) K2(ChaKChaE)2 [NaCl = 0 mM]; (H) K4(ChaKChaE)2 [NaCl = 100 mM]; (J) K2(AKAE)2 [NaCl = 1 M].
