Fig. 4.
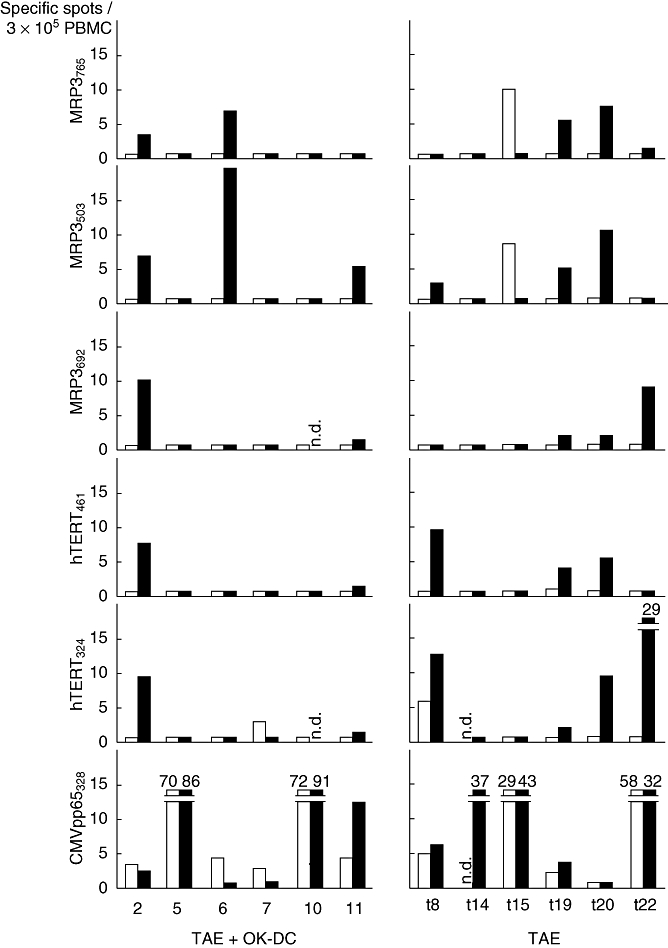
Immune responses to human leucocyte antigen (HLA-DR-)-A24-restricted peptide epitopes derived from tumour antigens in HLA-A24-positive patients treated with OK432-stimulated DCs during transcatheter hepatic arterial embolization (TAE) therapy (numbers 2, 5, 6, 7, 10 and 11) and HLA-A24-positive historical controls treated with TAE without dendritic cell (DC) transfer (numbers t8, t14, t15, t19, t20 and t22). Peripheral blood mononuclear cells (PBMCs) were obtained before (open bars) and 1 month after the infusion (solid bars), pulsed with the peptides derived from squamous cell carcinoma antigen recognized by T cells 2 (SART2), SART3, multi-drug resistance protein 3 (MRP3), alpha-fetoprotein (AFP), human telomerase reverse transcriptase (hTERT) and interferon (IFN)-γ production was quantitated by enzyme-linked immunospot (ELISPOT). Negative controls consisted of a human immunodeficiency virus (HIV) envelope-derived peptide (HIVenv584). Positive controls consisted of 10 ng/ml phorbol 12-myristate 13-acetate (PMA) or a cytomegalovirus (CMV) pp65-derived peptide (CMVpp65328). The number of specific spots was determined by subtracting the number of spots in the absence of antigen from the number of spots in its presence. T lymphocyte responses to the peptide epitopes were induced following TAE therapy, but no additional responses were observed after DC transfer. Numbers denote specific spots beyond the upper limit of y-axis; n.d., not determined.
