Table 1.
Preliminary study of the reactions between allenoate 1 and aldehydes 2a
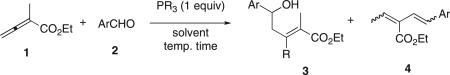 | ||||||
|---|---|---|---|---|---|---|
| Entry | Ar | R | Solvent | Time [h] | Temp [°C] | Yieldb [%] |
| 1 | C6H5 | C6H5 | Benzene | 24 | Reflux | 28 (3a) |
| 2 | C6H5 | C6H5 | Toluene | 24 | Reflux | 28 (3a) |
| 3 | C6H5 | C6H5 | EtOAc | 24 | Reflux | 28 (3a) |
| 4c | C6H5 | C6H5 | Neat | 2 | 160 | 25 (3a) |
| 5 | 4-CF3C6H4 | C6H5 | Benzene | 24 | Reflux | 33 (3b) |
| 6 | 4-NCC6H4 | C6H5 | Toluene | 24 | Reflux | 33 (3c) |
| 7 | 4-NCC6H4 | C6H5 | Toluene | 24 | rt | Trace (3c) |
| 8 | 4-NCC6H4 | C6H5 | MeOH | 24 | rt | 0 |
| 9 | 4-BrC6H4 | C6H5 | Benzene | 24 | Reflux | 21 (3d) |
| 10 | 3-HOC6H4 | C6H5 | Benzene | 24 | Reflux | 0 |
| 11c,d | 4-Me2NC6H4 | C6H5 | Toluene | 54 | 160 | 0 |
| 12c | 4-Me2NC6H4 | C6H5 | Neat | 48 | 160 | 0 |
| 13 | 4-NCC6H4 | 4-MeC6H4 | Toluene | 24 | Reflux | 21 (3e) |
| 14e | 4-NCC6H4 | C6H5 | Toluene | 24 | Reflux | 37 (4a) |
Allenic ester (1.2 mmol) in solvent (10 mL) was added slowly (over 2 h) via a syringe pump into the mixture of the other reaction components (1.0 mmol of aldehyde and 1.0 mmol of phosphine) in solvent (5 mL).
Isolated yield of product.
Reaction was performed in a sealed tube.
Total volume of solvent: 2 mL.
BF3·Et2O (10 mol %) was added to the reaction mixture.
