Table 2.
Vinylogous Wittig reactions between the allenoate 1 and the aldehyde 2aa
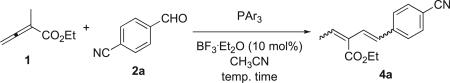 | ||||||
|---|---|---|---|---|---|---|
| Entry | 1 (equiv) | 2a (equiv) | PAr3 (equiv) | t [h] | T [°C] | Yieldb [%] |
| 1 | 1.2 | 1 | PPh3 (1) | 36 | 0 | 0 |
| 2 | 1.2 | 1 | PPh3 (1) | 36 | rtc | 30 |
| 3 | 2.5 | 1 | PPh3 (1) | 36 | rt | 30 |
| 4 | 1.2 | 1 | PPh3 (1) | 36 | 40 | 37 |
| 5 | 1.2 | 1 | PPh3 (1) | 30 | 80 | 42 |
| 6 | 1.2 | 5 | PPh3 (1) | 30 | 80 | 29 |
| 7 | 1.2 | 1 | PPh3 (5) | 30 | 80 | 55 |
| 8 | 1.2 | 1 | (4-FC6H4)3P (1) | 144 | rt | 47 |
Allenic ester in CH3CN (5 mL) was added in one portion to a mixture of the other components in CH3CN (10 mL).
Isolated yield.
rt=room temperature.
