Table 4.
Effects of various aldehyde substrates on the vinylogous Wittig transformationa
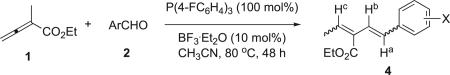 | ||||
|---|---|---|---|---|
| Entry | Ar | Product | Yieldb [%] | E/Z ratioc |
| 1 | 4-NCC6H4 | 4a | 63 | 5:2 |
| 2 | 3-NCC6H4 | 4b | 65 | 5:2 |
| 3 | 2-NCC6H4 | 4c | 60 | 5:1 |
| 4 | 4-O2NC6H4 | 4d | 51 | 4:1 |
| 5 | 3-O2NC6H4 | 4e | 57 | 2:1 |
| 6 | 2-O2NC6H4 | 4f | 50 | 7:2 |
| 7 | 3-FC6H4 | 4g | 49 | 5:2 |
Allenic ester in CH3CN (5 mL) was added in one portion to the mixture of the other components in CH3CN (10 mL).
Isolated yield.
Relating to the geometry around the newly formed double bond; the ratio was determined after integration of the signals of protons Ha and Hb in the 1H NMR spectrum of the crude reaction product.
