Abstract
Human adenovirus of strains subgenus F (AdV F) are the most common strains detected in acute gastroenteritis cases in developing countries. Subgenus F is represented by AdV serotype 40 (AdV-40) and AdV-41. Most of the reports have described the predominance of AdV-41 in acute gastroenteritis cases. To gain insight into the epidemiology and genetic variation of AdV-41 strains, we analyzed 1,053 stool specimens from children with diarrhea. Among them, 42 (4.0%) and 56 (5.3%) were positive for enteric adenovirus 40/41 by enzyme-linked immunosorbent assay (ELISA) and PCR, respectively. For 1,305 asymptomatic children, 9 (0.7%) and 22 (1.7%) samples were positive for enteric adenovirus 40/40 by ELISA and PCR, respectively. The age distribution revealed a higher frequency (90%) in children <24 months of age. AdV F infection was observed at a low frequency throughout the year, with an increased incidence occurring during February and March. Sequence analysis of one to three hypervariable regions (HVRs) of the hexon genes of 16 representative AdV-41 strains in this study confirmed circulation of a unique strain with genomic type cluster 1 (GTC1)/GTC2. However, sequence analysis of the fiber genes of these strains confirmed 15 amino acid deletions from the 15th repeat motif of the shaft region. The existence of two GTCs reflects the accumulation of amino acid mutations in the HVR of the hexon gene. The novel AdV-41 strain might follow the same infection pattern as AdV-40. There is no significant variation in the sequences of hexon and fiber genes among strains from symptomatic and asymptomatic children. Our data confirm the circulation of an AdV-41 strain with a novel pattern in Kolkata, India, among children below 5 years of age.
Diarrheal diseases are a major public health problem that particularly affect children in developing countries. Acute gastroenteritis is a global health problem and a major contributor to childhood morbidity and mortality reported worldwide (30). It has been reported that approximately 1.76 million deaths occur due to gastroenteritis among children (age, <5 years) worldwide (36). A number of bacterial, viral, and parasitic agents have been identified in patients with acute diarrhea (3, 13, 15, 21, 31, 54). Among the different enteric diarrheal viruses, rotaviruses are considered a major cause of severe gastroenteritis in infants and young children worldwide (35). The association of other enteric viruses, such as caliciviruses, astroviruses, and enteric adenoviruses (AdVs), with diarrhea has been reported in recent studies (1, 31, 41, 46) as well as a previous study (51). Human adenovirus belongs to the Mastadenovirus genus of the family Adenoviridae and has been implicated in acute respiratory, gastrointestinal, and urinary tract infections. To date, 52 human adenovirus serotypes have been identified and classified into six subgenera (subgenera A to F) on the basis of their biological and genetic characteristics (50). Among these subgenera, subgenus F (AdV F), representing adenovirus type 40 (AdV-40) and AdV-41, has been found to be associated with acute gastroenteritis and is responsible for 1 to 20% of the cases of diarrheal disease globally in both outpatients and hospitalized children (5, 24, 41, 42, 48). AdV-40 and AdV-41 primarily affect young children less than 2 years of age and occur throughout the year. The clinical characteristics include watery diarrhea accompanied by vomiting, low-grade fever, and mild dehydration. A distinct feature of AdV-40 and AdV-41 infections is the protracted diarrhea (means, 8.6 and 12.2 days, respectively).
AdV-40 and AdV-41 differ from all other adenoviruses, as they are difficult to cultivate in conventional cell cultures. Due to this, enzyme-linked immunosorbent assay (ELISA) and solid-phase immune electron microscopy are the methods primarily used for diagnosis. Species-specific PCR targeting human adenoviruses and restriction endonuclease digestion of the PCR amplicon have also facilitated the rapid and accurate diagnosis of adenovirus infection in clinical specimens during the past decade (2, 37).
In India, a few reports have described AdV F infection in children with gastroenteritis (40, 44), with AdV-41 predominating (49); however, there is no report from Kolkata, West Bengal, India, which is one of the areas of endemicity for diarrheal diseases. The main objective of the present study was to determine the prevalence of enteric adenoviruses as a cause of diarrhea among children <5 years of age from Kolkata and to perform a genetic analysis of AdV-41. Additionally, the seasonality of adenovirus infection from this region of India was also investigated.
MATERIALS AND METHODS
Study population, sample collection, and processing.
A total of 1,053 and 1,305 stool samples were collected from symptomatic and asymptomatic children (age, <5 years), respectively, from the B. C. Roy Memorial Hospital for Children and Infectious Diseases Hospital in Kolkata from December 2007 to December 2009. Inclusion criteria for symptomatic children included passing of three or more loose/watery stools within 24 h. The control population was healthy children from an urban population in the same region who had had no diarrhea during the 2 weeks preceding enrollment in the study. Written informed consent was obtained from the parents of the enrolled children. The study was approved by the institutional ethical committee. Aliquots of stool samples were stored at −20°C.
Extraction of viral genome.
Viral DNA was extracted from 200 μl of fecal suspension using an automated nucleic acid isolation system (NucliSens easyMAG; bioMérieux, Netherlands), according to the manufacturer's procedure.
Detection of adenovirus and enteric adenoviruses by ELISA.
Adenovirus was detected by use of a ProSpecT adenovirus ELISA kit (Oxoid, Cambridge, United Kingdom) following the manufacturer's procedure. The adenovirus-positive fecal samples were further screened for enteric adenovirus by use of a serotype-specific Adenoclone 40/41 ELISA kit (Meridian Bioscience Inc., Cincinnati, OH).
Detection of enteric adenovirus by PCR.
Nucleic acids of 101 ELISA-positive samples were used for identification of AdV-40 and AdV-41. The hexon gene was amplified using primers AdFhex(+) (5′-GCCACCGATACCTACTTCAGCCTG-3′) and AdFhex(−) (5′-GGCAGTGCCGGAGTAGGGTTTAAA-3′), followed by DNA sequencing of the ∼261-bp PCR product. PCR was performed at 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min and a final extension at 72°C for 7 min and was then held at 4°C. The PCR products were electrophoresed in a 1.5% agarose gel.
Serotyping of adenovirus by PCR and sequence analysis.
AdV-41 was further subtyped by using specific primers S29(+) (5′-GCCAGCACRTWCTTTGACAT-3′) and S52(−) (5′-CCCATGTTGCCAGTGCTGTTGTARTACA-3′) of three hypervariable regions (HVRs) of the hexon gene (24). PCR was performed under the same conditions mentioned above but with annealing at 55°C. The primers gave an amplicon of 668 bp. The partial shaft region of the fiber genes was also amplified using primers AdFfib(+) (5′-ACTTAATGCTGACACGGGCAC-3′) and AdFfib(−) (5′-TAATGTTTGTGTTACTCCGCTC-3′) for phylogenetic analysis (16, 53). PCR was performed in a 0.2-ml reaction tube in a total volume of 25 μl containing 5 μl of 5× Go Taq reaction buffer, 0.5 μl of a 10 mM deoxynucleoside triphosphate (dNTP) mixture, 0.2 μl Go Taq polymerase (5 U/μl), 1 μl of each primer at 10 pM, and 2 μl of nucleic acid extract, according to the manufacturer's instructions (Promega Corporation, Madison, WI). The primers gave an amplicon of 561 bp.
Nucleotide sequence and phylogenetic analysis.
For sequencing, the PCR amplicons were purified using a QIAquick PCR purification kit (Qiagen, Netherlands) and directly sequenced using a BigDye DNA sequencing kit in an automated DNA sequencer (ABI Prism 3100; Applied Biosystems, Foster City, CA). Both the forward and reverse primers were used for sequencing the PCR products. The nucleotide and deduced amino acid sequences of the hexon gene and the partial shaft region of the fiber gene were compared with those of prototype and other reported strains of enteric adenoviruses using ClustalX software. The following reference strains of human adenovirus (with their GenBank accession numbers in parentheses) were selected: for the hexon genes, D1-VN47 (AB103341), D4-VN28 (AB103342), D12-JP3171 (AB103343), D22-Km079 (AB103344), D26-JP31106 (AB103345), D27-JP2149 (AB103346), D25-Ks35 (AB103347), D28-VN1020 (AB103348), and TAK (X51783), with strain Dugan (X51782) used as the outgroup, and for the fiber genes, strains TAK (X16583) and M60327 (FB585), with strains Dugan (L19443) and GU245891 used as the outgroup. Phylogenetic analyses was done using the MEGA (version 4) software package (47). Trees were constructed by the neighbor-joining (NJ) method with 1,000 bootstrap replications in the ClustalW program and drawn by use of the NJ plot program (http://pbil.univ-lyon1.fr/software/njplot.html).
Nucleotide sequence accession numbers.
The nucleotide sequence data for the hexon and fiber genes were submitted to GenBank (DDBJ, Japan) and were assigned accession numbers HQ005278 to HQ005290 and HQ010340 to HQ010358, respectively.
RESULTS
Prevalence of adenovirus infection among children in Kolkata.
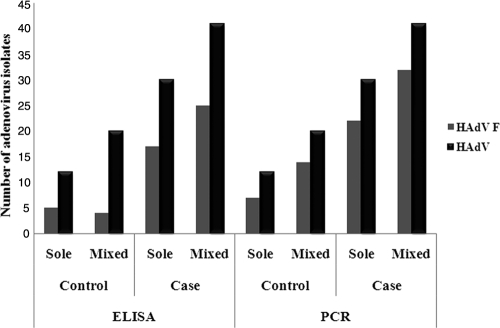
Among 1,053 children (age, <5 years) with diarrhea, 69 (6.5%, 69/1,053) were adenovirus ELISA positive, and of these, 42 (4.0%, 42/1,053) were positive for enteric adenovirus serotype 40/41. In 1,305 asymptomatic children, 32 (2.5%, 32/1,305) were adenovirus ELISA positive, and of these, only 9 (0.7%, 9/1,305) were positive for enteric adenovirus 40/41. For further confirmation of enteric adenovirus subtypes, all the adenovirus-specific-ELISA-positive samples were subjected to PCR with hexon gene-specific primers, followed by sequencing, and were analyzed by BLAST analysis of the nucleotide sequences. PCR followed by sequencing of 101 (69 + 32) samples revealed a higher frequency of enteric adenovirus 40/41 (56 [81.1%, 56/69] and 22 [68.7%, 22/32] in symptomatic and asymptomatic AdV-positive stool samples, respectively) than the AdV-40/41-specific ELISA (Fig. 1). Of the 78 enteric adenovirus subtype F isolates, 53 (68%, 53/78) and 25 (32%, 25/78) belonged to enteric AdV-41 and AdV-40, respectively. Among the 101 samples, serotype 41 was more prevalent (52.5%), followed by serotype 40 (24.7%), serotype 15 (7%), serotype 5 (3%), and serotype 12 (2%). Overall, the prevalence rates of enteric AdV-40/41 have been identified to be 50.5% (51/101) and 77.2% (78/101) by ELISA and PCR, respectively, in this study.
Fig. 1.
Comparison of the detection of enteric adenovirus 40/41 by ELISA and PCR followed by sequencing from stool samples from children in Kolkata, India, collected from December 2007 through December 2009.
Correlation of adenovirus infection with age and seasonality.
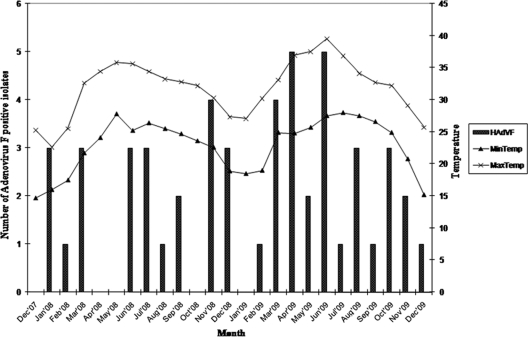
To determine whether seasonal changes play a role in the prevalence of adenovirus, the data were analyzed on a monthly basis for 2 years. Enteric AdV infection was predominant in February and March in both years, though sporadic cases were observed throughout the year (Fig. 2). In both symptomatic and asymptomatic cases, enteric adenovirus infection was mostly detected in children of less than 2 years of age. The median age of children with AdV-40 infection was 8 months (range, 3 to 20 months), whereas it was 15 months (range, 5 to 55 months) for AdV-41-positive cases.
Fig. 2.
Seasonal distribution of enteric adenovirus 40/41 during the period from December 2007 to December 2009.
Nucleotide sequence and phylogenetic analysis of hexon and fiber genes of AdV-41 strains.
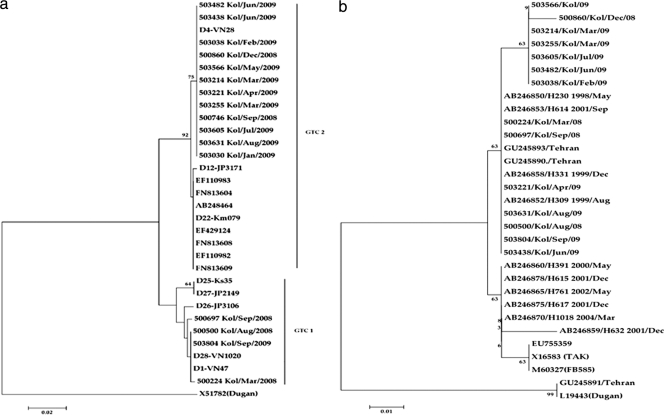
For genetic analysis, one to three HVRs of 16 representative strains of AdV-41 obtained in the present study (identified during the entire span of the study) were sequenced. Of the 16 strains, 2 were taken from asymptomatic children for comparison of sequences. A phylogenetic tree based on the nucleotide sequences of the hexon gene was subsequently constructed. This analysis revealed the presence of two genomic type clusters (GTCs) in the population (Fig. 3a). Twelve out of 16 sequences (December 2008 to August 2009) belonged to the GTC2 genotype, with the sequences being similar to the D4 genome type sequence (24). Three of 16 strains obtained from December 2007 to November 2008 and 1 strain obtained in September 2009 belonged to GTC1, and the sequences had a very high degree of similarity with those of the D1 and D28 genome types (24). The nucleotide sequence identities between the two GTCs in the hexon gene ranged from 91.2 to 100%. For the hexon gene, the nucleotide sequence identities among themselves were 99.7% to 100% for GTC1 and 100% for GTC2. This is the first report which shows the prevalence of AdV-41 of GTC1 (D1 and D28) and GTC2 (D4) in Kolkata.
Fig. 3.
Phylogenetic tree based on the nucleotide sequence of the HVR (1 to 3) of the hexon gene (a) and the nucleotide sequence of the shaft region (partial) of the fiber gene (b) of representative AdV-41 strains isolated from children in Kolkata, India, from December 2007 through December 2009. The phylogenetic trees were constructed by the neighbor-joining method with 1,000 bootstrap replications in the Clustal W program (http://www.ddbj.nig.ac.jp/search/clustalw-j.html). The numbers at internal nodes indicate the bootstrap values. The designations indicate the strain name/city name/month of infection/year of infection for the strains detected in this study. GTC1 and GTC2 denote genome type clusters in the hexon gene, as described by Li et al. (24). For the hexon gene, the following reference strains of human adenovirus (GenBank accession numbers are indicated in parentheses) were selected from GenBank: for hexon genes, D1-VN47 (AB103341), D4-VN28 (AB103342), D12-JP3171 (AB103343), D22-Km079 (AB103344), D26-JP31106 (AB103345), D27-JP2149 (AB103346), D25-Ks35 (AB103347), D28-VN1020 (AB103348), and TAK (X51783), with strain Dugan (X51782) used as the outgroup, and for the fiber gene, TAK (X16583) and M60327 (FB585), with strains Dugan (L19443) and GU245891 used as the outgroup.
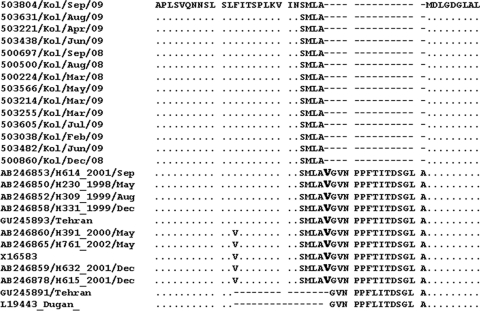
A phylogenetic tree based on the nucleotide sequences of the shaft region of the fiber gene revealed clustering of all the 14 representative strains along with the strains of GTC2 (Fig. 3b). The nucleotide sequence identity was found to be 99.5 to 99.7% among themselves and 88% to 88.7% with the prototype strain (TAK). ClustalW analysis of the deduced amino acid sequences showed 15 amino acid deletions in all the Kolkata strains sequenced in this study (Fig. 4). This deletion of the 15 amino acids was confined to 15th repeat motif in the fiber gene shaft region.
Fig. 4.
Alignment of the deduced amino acid sequences of the fiber gene shaft region from 14 representative AdV-41 strains. One amino acid that overlaps with the sequence of the reference AdV-41 strain has been shown in boldface.
DISCUSSION
Molecular epidemiological studies are increasingly important in the field of clinical adenovirus research (24, 34). Epidemiological studies of adenovirus in infants and children with acute gastroenteritis have shown its importance in both developed and developing countries (6, 10, 11, 17, 18, 23, 31, 39, 43, 49). Next to rotavirus, enteric adenovirus has been shown to be the most common cause of pediatric viral gastroenteritis in Kolkata (31). In Japan, the high incidence (8%) of adenovirus infection made adenovirus a second common agent of acute gastroenteritis (41). In the present study, we analyzed stool samples by ELISA and by PCR and sequence analysis and observed enteric AdV infections at rates of 4% (42/1,053 samples) and 5.3% (56/1,053), respectively, in symptomatic children and 0.9% (9/1,305) and 1.7% (22/1,305), respectively, in asymptomatic children. The actual prevalence would have been slightly higher if PCR instead of ELISA was used as the first step in screening, but screening 2,358 samples by PCR was both time-consuming and costly. The results of our study are consistent with the rates of detection of adenoviruses among acute gastroenteritis cases in other developed and developing countries, which range from 2.3 to 38%, although the methods of detection are not comparable (11, 22, 25, 27, 28, 32).
The age distribution of individuals with AdV-40/41 infection confirmed that 90% of the positive cases occurred in children under 2 years of age. Even though the prevalence rate was low in asymptomatic children, in both the symptomatic and asymptomatic groups of children, children under 2 years of age were found to be more susceptible to AdV-40/41 infection. Our result is consistent with the findings of epidemiological studies on enteric adenoviruses worldwide, in which adenovirus infection associated with acute gastroenteritis is reported predominantly in infants and young children (1, 7, 10, 18, 29, 33, 38, 41). As reported in many studies, we also observed that the frequency of AdV-40/41 infection was high but sporadic during winter months (4, 9, 11, 17, 45). Diarrheal outbreaks due to AdV-40/41 infection were reported to occur from November to January (10, 41).
Very little information on the molecular characterization of adenovirus serotypes is available. Verma et al. (49) had identified AdV-40, AdV-41, and AdV-31 to be the most prevalent adenovirus serotypes (7.7%) associated with acute gastroenteritis in infants and children in western India, but the strains were not further characterized. The prevalence of species D human adenoviruses was found to be high in Kenyan children with diarrhea (26). In this study, PCR-based sequence analysis of genomic DNA of adenoviruses confirmed that serotype 41 is more prevalent (68%), followed by serotype 40 (32%). Serotypes 15 and 5 were also reported to be associated with diarrhea in the region, but their prevalence was very low. To our knowledge, this is the first report of the detection of an association of serotypes 15 and 5 with diarrhea. However, mixed infections with other pathogens as the cause of diarrhea in this case cannot be ruled out. In previous reports, a predominance of AdV-41 over AdV-40 has been shown (10, 11, 14, 19, 39, 41). This trend may be associated with the higher pathogenicity of AdV-41 in these communities (24, 52). In contrast, studies in rural Bangladesh, the Netherlands, and Germany showed that the frequencies of these two serotypes were almost the same (8, 17). Long-term monitoring for these serotypes in the same population where adenovirus is endemic may give an answer regarding serotype drifts, if any exist.
The phylogenetic tree for the 1 to 3 HVRs of the hexon gene confirmed the presence of two genomic type clusters: GTC1 and GTC2 (24). AdV-41 has already been characterized by sequencing of the hexon gene, which classifies it into two genomic type clusters, GTC1 and GTC2. GTC1 includes strains D1, D25, D26, D27, and D28 and GTC2 contains strains D4, D12, and D22 (24, 52). The existence of two GTCs reflects the accumulation of amino acid mutations in the HVRs of the hexon gene. This is the first report from India demonstrating circulation of two distinct AdV-41 genome types in the same population. It is very interesting to note that GTC2 predominated from December 2008 to August 2009, while GTC1 predominated from December 2007 to November 2008 and then from September 2009 to December 2009. Interestingly, GTC1 clustered with D1 and D28 (GenBank accession numbers AB103341 and AB103348, respectively), whereas all GTC2 strains clustered with D4 type strains (GenBank accession numbers AB103342). Li et al. (24) first reported the same pattern of hexon gene sequence clustering in AdV-41 strains identified from stool samples from Vietnamese children, which was also seen in the present study. Our study has a limitation because it did not use the antigen-specific monoclonal antibody ELISA used by Li et al. (24). The sequences of the hexon genes of two strains of AdV-41 from stool samples from asymptomatic children revealed that the sequences were identical to those of the hexon genes of strains from stool samples from symptomatic children.
For the correlation of the GTC of the hexon gene with that of the fiber gene, a phylogenetic tree was constructed for the fiber gene, which confirmed that most representative strains cluster and are associated with the GTC2 hexon gene. Fukuda et al. (12) reported one strain with a GTC1 (D1 and D28) hexon gene which clustered with strains with a GTC2 (D4) fiber gene sequence, and this could be due to recombination between adenoviruses of the same type. Four strains with a hexon gene of GTC1 (D1 and D28) but a fiber gene of GTC2 were identified in this study, indicating possible recombination among cocirculating strains. Interestingly, analysis of the amino acid sequences of the fiber gene revealed deletion of 15 amino acids in the Kolkata strains. There are 22 repeat motifs in the shaft region of the AdV-41 fiber polypeptide, where there are 21 motifs in the shaft region of the AdV-40 fiber polypeptide. In the present study, deletion of the 15th repeat motif of AdV-41 was observed. All the AdV-40 Kolkata strains had 21 repeat motifs (data not shown) and a 15-amino-acid deletion from the 14th repeat motif of the fiber shaft polypeptide, as reported above. The coding region of the shaft region was intact, as the entire 15-amino-acid coding region from both AdV-40 and AdV-41 had been excised from the sequence. This deletion of the 15th repeat motif from novel AdV-41 strains decreases the length of the fiber, and so it became the same size as the fiber of AdV-40. It seems that AdV-40 and this novel AdV-41 strain circulating in the eastern part of India use the same mechanism of interaction with the host receptor. This deletion has been evolutionarily conserved in the strains circulating in Kolkata. This diversity of AdV-41 has been previously reported, where strains Ad41/D6/17951/Netherlands/83 and Ad41/D8/N7761/Canada/81 had similar deletions (20). Unfortunately, the genome sequences of these strains are not available in GenBank for comparison with the fiber and hexon gene sequences of the Kolkata strains. The fiber shaft has a triple β-spiral motif which is stable yet flexible. It has been speculated that a shortened fiber length may also have implications in improved replication (20) and/or an association with pathogenicity, which has yet to be proved.
In conclusion, our findings suggest that the novel AdV-41 strain is an important pathogen in causing acute gastroenteritis among children in Kolkata. To our knowledge, this is the first report on genetic characterization of AdV-41 strains, revealing the presence of unique 15 amino acids deletion in the fiber gene, irrespective of the GTC1 or GTC2 genotype.
ACKNOWLEDGMENTS
This study was supported in part by the Indian Council of Medical Research, Council of Scientific and Industrial Research, New Delhi, India, and Global Multicentric Study, University of Maryland, Baltimore, MD.
We acknowledge the help of Mehuli Sarkar, Anurodh Shankar Agarwal, and Goutam Chowdhury for the laboratory work and Manash Roy for processing the data.
We declare that no conflicts of interest exist.
Footnotes
Published ahead of print on 1 December 2010.
REFERENCES
- 1. Akihara S., et al. 2005. Existence of multiple outbreaks of viral gastroenteritis among infants in a day care center in Japan. Arch. Virol. 150:2061–2075 [DOI] [PubMed] [Google Scholar]
- 2. Allard A., Albinsson B., Wadell G. 2001. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J. Clin. Microbiol. 39:498–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baqui A. H., et al. 1992. Epidemiological and clinical characteristics of acute and persistent diarrhoea in rural Bangladeshi children. Acta Paediatr. Suppl. 381:15–21 [DOI] [PubMed] [Google Scholar]
- 4. Barnes G. L., Uren E., Stevens K. B., Bishop R. F. 1998. Etiology of acute gastroenteritis in hospitalized children in Melbourne, Australia, from April 1980 to March 1993. J. Clin. Microbiol. 36:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandt C. D., et al. 1985. Adenoviruses and pediatric gastroenteritis. J. Infect. Dis. 151:437–443 [DOI] [PubMed] [Google Scholar]
- 6. Broor S., et al. 2007. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS One 2:e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiba S., et al. 1983. Outbreak of infantile gastroenteritis due to type 40 adenovirus. Lancet ii:954–957 [DOI] [PubMed] [Google Scholar]
- 8. de Jong J. C., et al. 1983. Candidate adenoviruses 40 and 41. Fastidious adenoviruses from human infant stool. J. Med. Virol. 11:215–231 [DOI] [PubMed] [Google Scholar]
- 9. Dennehy P. H., et al. 2001. A prospective case-control study of the role of astrovirus in acute diarrhea among hospitalized young children. J. Infect. Dis. 184:10–15 [DOI] [PubMed] [Google Scholar]
- 10. Dey S. K., et al. 2009. Molecular epidemiology of adenovirus infection among infants and children with acute gastroenteritis in Dhaka City, Bangladesh. Infect. Genet. Evol. 9:518–522 [DOI] [PubMed] [Google Scholar]
- 11. Filho E. P., et al. 2007. Adenoviruses associated with acute gastroenteritis in hospitalized and community children up to 5 years old in Rio de Janeiro and Salvador, Brazil. J. Med. Microbiol. 56:313–319 [DOI] [PubMed] [Google Scholar]
- 12. Fukuda S., Kuwayama M., Takao S., Shimazu Y., Miyazaki K. 2006. Molecular epidemiology of subgenus F adenoviruses associated with pediatric gastroenteritis during eight years in Hiroshima Prefecture as a limited area. Arch. Virol. 151:2511–2517 [DOI] [PubMed] [Google Scholar]
- 13. Germani Y., et al. 1994. Two-year study of endemic enteric pathogens associated with acute diarrhea in New Caledonia. J. Clin. Microbiol. 32:1532–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grimwood K., Carzino R., Barnes G. L., Bishop R. F. 1995. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981 to 1992. J. Clin. Microbiol. 33:131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerrant R. L., et al. 1983. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J. Infect. Dis. 148:986–997 [DOI] [PubMed] [Google Scholar]
- 16. Hussain M. A., et al. 1996. Comparison of primer sets for detection of fecal and ocular adenovirus infection using the polymerase chain reaction. J. Med. Virol. 49:187–194 [DOI] [PubMed] [Google Scholar]
- 17. Jarecki-Khan K., Tzipori S. R., Unicomb L. E. 1993. Enteric adenovirus infection among infants with diarrhea in rural Bangladesh. J. Clin. Microbiol. 31:484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jarecki-Khan K., Unicomb L. E. 1992. Seroprevalence of enteric and nonenteric adenoviruses in Bangladesh. J. Clin. Microbiol. 30:2733–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamel A. H., Ali M. A., El-Nady H. G., de Rougemont A., Pothier P., Belliot G. 2009. Predominance and circulation of enteric viruses in the region of Greater Cairo, Egypt. J. Clin. Microbiol. 47:1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kidd A. H., Erasmus M. J., Tiemessen C. T. 1990. Fiber sequence heterogeneity in subgroup F adenoviruses. Virology 179:139–150 [DOI] [PubMed] [Google Scholar]
- 21. Kosek M., Bern C., Guerrant R. L. 2003. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 81:197–204 [PMC free article] [PubMed] [Google Scholar]
- 22. Lew J. F., et al. 1991. Astrovirus and adenovirus associated with diarrhea in children in day care settings. J. Infect. Dis. 164:673–678 [DOI] [PubMed] [Google Scholar]
- 23. Li C. S., Chan P. K., Tang J. W. 2009. Prevalence of diarrhea viruses in hospitalized children in Hong Kong in 2008. J. Med. Virol. 81:1903–1911 [DOI] [PubMed] [Google Scholar]
- 24. Li L., et al. 2004. Characterization of adenovirus type 41 isolates from children with acute gastroenteritis in Japan, Vietnam, and Korea. J. Clin. Microbiol. 42:4032–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu C., et al. 2006. Identification of viral agents associated with diarrhea in young children during a winter season in Beijing, China. J. Clin. Virol. 35:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magwalivha M., et al. 2010. High prevalence of species D human adenoviruses in fecal specimens from urban Kenyan children with diarrhea. J. Med. Virol. 82:77–84 [DOI] [PubMed] [Google Scholar]
- 27. Marie-Cardine A., et al. 2002. Epidemiology of acute viral gastroenteritis in children hospitalized in Rouen, France. Clin. Infect. Dis. 34:1170–1178 [DOI] [PubMed] [Google Scholar]
- 28. McIver C. J., et al. 2001. Diagnosis of enteric pathogens in children with gastroenteritis. Pathology 33:353–358 [PubMed] [Google Scholar]
- 29. Moore P., Steele A. D., Lecatsas G., Alexander J. J. 1998. Characterisation of gastro-enteritis-associated adenoviruses in South Africa. S. Afr. Med. J. 88:1587–1592 [PubMed] [Google Scholar]
- 30. Murray C. J., Lopez A. D. 1997. Mortality by cause for eight regions of the world; Global Burden of Disease study. Lancet 349:1269–1276 [DOI] [PubMed] [Google Scholar]
- 31. Nair G. B., et al. 2010. Emerging trends in the etiology of enteric pathogens as evidenced from an active surveillance of hospitalized diarrhoeal patients in Kolkata, India. Gut Pathog. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakanishi K., et al. 2009. Detection of enteric viruses in rectal swabs from children with acute gastroenteritis attending the pediatric outpatient clinics in Sapporo, Japan. J. Clin. Virol. 46:94–97 [DOI] [PubMed] [Google Scholar]
- 33. Oh D. Y., Gaedicke G., Schreier E. 2003. Viral agents of acute gastroenteritis in German children: prevalence and molecular diversity. J. Med. Virol. 71:82–93 [DOI] [PubMed] [Google Scholar]
- 34. Okitsu-Negishi S., Nguyen T. A., Phan T. G., Ushijima H. 2004. Molecular epidemiology of viral gastroenteritis in Asia. Pediatr. Int. 46:245–252 [DOI] [PubMed] [Google Scholar]
- 35. Parashar U. D., Gentseh J. R., Glass R. I. 1998. Rotavirus. Emerg. Infect. Dis. 4:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parashar U. D., Hummelman E. G., Bresee J. S., Miller M. A., Glass R. I. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pring-Akerblom P., Trijssenaar F. E., Adrian T., Hoyer H. 1999. Multiplex polymerase chain reaction for subgenus-specific detection of human adenoviruses in clinical samples. J. Med. Virol. 58:87–92 [PubMed] [Google Scholar]
- 38. Saderi H., et al. 2002. Incidence of enteric adenovirus gastroenteritis in Iranian children. J. Clin. Virol. 24:1–5 [DOI] [PubMed] [Google Scholar]
- 39. Sdiri-Loulizi K., et al. 2009. Molecular epidemiology of human astrovirus and adenovirus serotypes 40/41 strains related to acute diarrhea in Tunisian children. J. Med. Virol. 81:1895–1902 [DOI] [PubMed] [Google Scholar]
- 40. Shetty M., Brown T. A., Kotian M., Shivananda P. G. 1995. Viral diarrhoea in a rural coastal region of Karnataka India. J. Trop. Pediatr. 41:301–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shimizu H., et al. 2007. An outbreak of adenovirus serotype 41 infection in infants and children with acute gastroenteritis in Maizura City, Japan. Infect. Genet. Evol. 7:279–284 [DOI] [PubMed] [Google Scholar]
- 42. Shinozaki T., et al. 1991. Epidemiology of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children in the Tokyo area. Scand. J. Infect. Dis. 23:543–547 [DOI] [PubMed] [Google Scholar]
- 43. Simpore J., et al. 2009. Aetiology of acute gastro-enteritis in children at Saint Camille Medical Centre, Ouagadougou, Burkina Faso. Pak. J. Biol. Sci. 12:258–263 [DOI] [PubMed] [Google Scholar]
- 44. Singh P. B., Sreenivasan M. A., Pavri K. M. 1989. Viruses in acute gastroenteritis in children in Pune, India. Epidemiol. Infect. 102:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soares C. C., et al. 2002. Prevalence of enteric adenoviruses among children with diarrhea in four Brazilian cities. J. Clin. Virol. 23:171–177 [DOI] [PubMed] [Google Scholar]
- 46. Sumi A., Kobayarhi N., Ontomo N. 2005. Proportion of sporadic gastroenteritis cases caused by rotavirus, norovirus, adenovirus and bacteria in Japan from Jan 2000 to Dec 2003. Microbiol. Immunol. 49:745–756 [DOI] [PubMed] [Google Scholar]
- 47. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 48. Uhnoo I., Wadell G., Svensson L., Johansson M. E. 1984. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J. Clin. Microbiol. 20:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Verma H., Chitambar S. D., Varanasi G. 2009. Identification and characterization of enteric adenoviruses in infants and children hospitalized for acute gastroenteritis. J. Med. Virol. 81:60–64 [DOI] [PubMed] [Google Scholar]
- 50. Wadell G. 1984. Molecular epidemiology of human adenoviruses. Curr. Top. Microbiol. Immunol. 110:191–220 [DOI] [PubMed] [Google Scholar]
- 51. Wigand R., Adrian T. H., Bricoutt F. 1987. A new human adenovirus of subgroup D: candidate adenovirus type 42. Arch. Virol. 94:283–286 [DOI] [PubMed] [Google Scholar]
- 52. Wood D. J., de Jong J. C., Bijlsma K., van der Avoort H. G. 1989. Development and evaluation of monoclonal antibody-based immune electron microscopy for diagnosis of adenovirus types 40 and 41. J. Virol. Methods 25:241–250 [DOI] [PubMed] [Google Scholar]
- 53. Xu W., McDonough M. C., Erdmann D. D. 2000. Species specific identification of human adenoviruses by a multiplex PCR assay. J. Clin. Microbiol. 38:4114–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamashiro T., et al. 1998. Etiological study of diarrheal patients in Vientiane, Lao People's Democratic Republic. J. Clin. Microbiol. 36:2195–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]