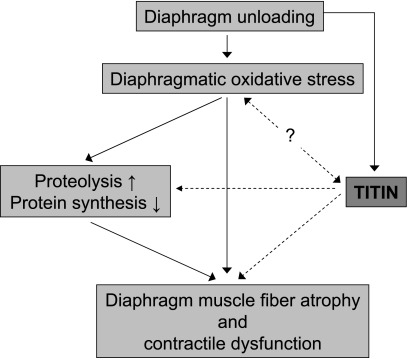
Fig. 3.
Schematic illustrating the potential role of titin in the development of diaphragm dysfunction during controlled mechanical ventilation. Prolonged mechanical ventilation and the concomitant diaphragm unloading results in diaphragm muscle fiber atrophy and contractile dysfunction. The current dogma regarding the pathogenesis of the diaphragmatic changes upon unloading includes key roles for diaphragmatic oxidative stress and increased proteolysis (through activation of the ubiquitin-proteasome pathway, calpains, and capases) and decreased protein synthesis (for a review see Ref. 46). Recent studies suggest that titin's properties respond to changes in muscle loading and that it acts as a mechanosensor that regulates the muscle's trophic status by regulation of protein expression and degradation. Furthermore, although little information is available regarding the effect of titin signaling on oxidative stress and vice versa, previous work suggests that titin's stiffness can be modulated by oxidative modification (33). We propose that titin, through its mechanosensing and signaling, contributes to the development of diaphragm contractile dysfunction and atrophy during mechanical ventilation-induced diaphragm unloading. Solid lines, established pathways involved in the pathogenesis of ventilator-induced diaphragm weakness; dashed lines, the novel titin-based pathways proposed in this Perspective.