Abstract
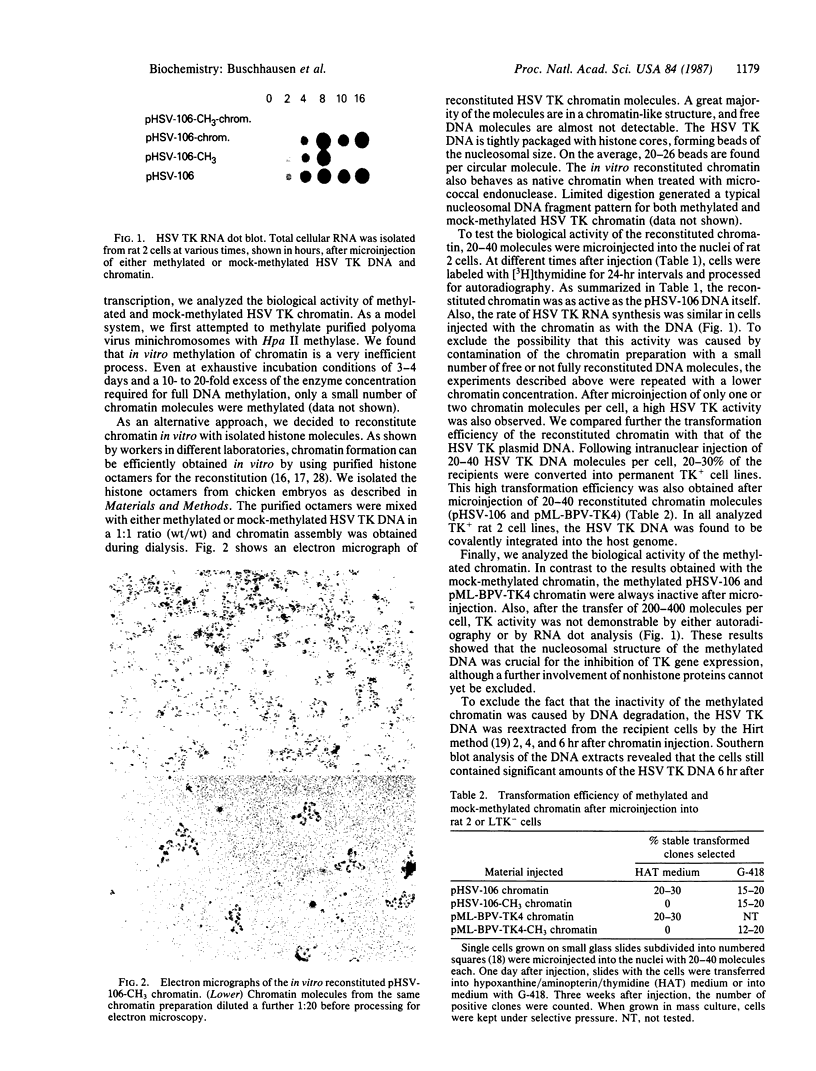
Inhibition of herpes simplex virus (HSV) thymidine kinase (TK) gene transcription (pHSV-106, pML-BPV-TK4) by DNA methylation is an indirect effect, which occurs with a latency period of approximately equal to 8 hr after microinjection of the DNA into TK- rat 2 and mouse LTK- cells. We have strong evidence that chromatin formation is critical for the transition of the injected DNA from methylation insensitivity to methylation sensitivity. Chromatin was reconstituted in vitro by using methylated and mock-methylated HSV TK DNA and purified chicken histone octamers. After microinjection, the methylated chromatin was always biologically inactive, as tested by autoradiography of the cells after incubation with [3H]thymidine and by RNA dot blot analysis. However, in transformed cell lines, reactivation of the methylated chromatin occurred after treatment with 5-azacytidine. Furthermore, integration of the TK chromatin into the host genome is not required to block expression of the methylated TK gene. Mouse cells that contained the pML-BPV-TK4 chromatin permanently in an episomal state also did not support TK gene expression as long as the TK DNA remained methylated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsoum J., Berg P. Simian virus 40 minichromosomes contain torsionally strained DNA molecules. Mol Cell Biol. 1985 Nov;5(11):3048–3057. doi: 10.1128/mcb.5.11.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Buschhausen G., Graessmann M., Graessmann A. Inhibition of herpes simplex thymidine kinase gene expression by DNA methylation is an indirect effect. Nucleic Acids Res. 1985 Aug 12;13(15):5503–5513. doi: 10.1093/nar/13.15.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., McGhee J. D. Structure of the 30 nm chromatin fiber. Cell. 1986 Feb 14;44(3):375–377. doi: 10.1016/0092-8674(86)90456-3. [DOI] [PubMed] [Google Scholar]
- Gilmour D. S., Pflugfelder G., Wang J. C., Lis J. T. Topoisomerase I interacts with transcribed regions in Drosophila cells. Cell. 1986 Feb 14;44(3):401–407. doi: 10.1016/0092-8674(86)90461-7. [DOI] [PubMed] [Google Scholar]
- Glikin G. C., Blangy D. In vitro transcription by Xenopus oocytes RNA polymerase III requires a DNA topoisomerase II activity. EMBO J. 1986 Jan;5(1):151–155. doi: 10.1002/j.1460-2075.1986.tb04189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A., Bumke-Vogt C., Buschhausen G., Bauer M., Graessmann M. SV40 chromatin structure is not essential for viral gene expression. FEBS Lett. 1985 Jan 1;179(1):41–45. doi: 10.1016/0014-5793(85)80187-3. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Topp W. C., Botchan M. Retransformation of a simian virus 40 revertant cell line, which is resistant to viral and DNA infections, by microinjection of viral DNA. J Virol. 1979 Dec;32(3):989–994. doi: 10.1128/jvi.32.3.989-994.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A. Microinjection of tissue culture cells. Methods Enzymol. 1983;101:482–492. doi: 10.1016/0076-6879(83)01033-2. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A. Simian virus 40 cRNA is processed into functional mRNA in microinjected monkey cells. EMBO J. 1982;1(9):1081–1088. doi: 10.1002/j.1460-2075.1982.tb01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A., Wagner H., Werner E., Simon D. Complete DNA methylation does not prevent polyoma and simian virus 40 virus early gene expression. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6470–6474. doi: 10.1073/pnas.80.21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Ziechmann C., Graessmann A. Methylation of the SV40 HpaII site does not affect late viral gene expression in microinjected tissue culture cells. FEBS Lett. 1984 Jul 23;173(1):151–154. doi: 10.1016/0014-5793(84)81036-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Tjian R. Sp1 binds to promoter sequences and activates herpes simplex virus 'immediate-early' gene transcription in vitro. Nature. 1985 Sep 12;317(6033):179–182. doi: 10.1038/317179a0. [DOI] [PubMed] [Google Scholar]
- Keshet I., Lieman-Hurwitz J., Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986 Feb 28;44(4):535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Klug A. The nucleosome. Sci Am. 1981 Feb;244(2):52–64. doi: 10.1038/scientificamerican0281-52. [DOI] [PubMed] [Google Scholar]
- Lindsey G. G., Thompson P., Pretorius L., Purves L. R., von Holt C. Octamer reconstitution from acid-extracted chicken erythrocyte histones. FEBS Lett. 1983 May 8;155(2):301–305. doi: 10.1016/0014-5793(82)80625-x. [DOI] [PubMed] [Google Scholar]
- Luchnik A. N., Bakayev V. V., Zbarsky I. B., Georgiev G. P. Elastic torsional strain in DNA within a fraction of SV40 minichromosomes: relation to transcriptionally active chromatin. EMBO J. 1982;1(11):1353–1358. doi: 10.1002/j.1460-2075.1982.tb01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Berg L., Weiher H., Botchan M. Bovine papilloma virus contains an activator of gene expression at the distal end of the early transcription unit. Mol Cell Biol. 1983 Jun;3(6):1108–1122. doi: 10.1128/mcb.3.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias P. D., Bernard H. U., Scott A., Brady G., Hashimoto-Gotoh T., Schütz G. A bovine papilloma virus vector with a dominant resistance marker replicates extrachromosomally in mouse and E. coli cells. EMBO J. 1983;2(9):1487–1492. doi: 10.1002/j.1460-2075.1983.tb01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L. Functional relationships between transcriptional control signals of the thymidine kinase gene of herpes simplex virus. Cell. 1982 Dec;31(2 Pt 1):355–365. doi: 10.1016/0092-8674(82)90129-5. [DOI] [PubMed] [Google Scholar]
- Melero J. A. Isolation and cell cycle analysis of temperature-sensitive mutants from Chinese hamster cells. J Cell Physiol. 1979 Jan;98(1):17–30. doi: 10.1002/jcp.1040980104. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Chromatin assembly in Xenopus oocytes: in vivo studies. Cell. 1984 May;37(1):21–32. doi: 10.1016/0092-8674(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Sariban E., Wu R. S., Erickson L. C., Bonner W. M. Interrelationships of protein and DNA syntheses during replication of mammalian cells. Mol Cell Biol. 1985 Jun;5(6):1279–1286. doi: 10.1128/mcb.5.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Wigmore D. J. Sites in simian virus 40 chromatin which are preferentially cleaved by endonucleases. Cell. 1978 Dec;15(4):1511–1518. doi: 10.1016/0092-8674(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Thoma F., Brubaker J. M. Chromatin reconstituted from tandemly repeated cloned DNA fragments and core histones: a model system for study of higher order structure. Cell. 1985 Oct;42(3):799–808. doi: 10.1016/0092-8674(85)90276-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Host-specificities of papillomavirus, Moloney murine sarcoma virus and simian virus 40 enhancer sequences. EMBO J. 1983;2(7):1193–1199. doi: 10.1002/j.1460-2075.1983.tb01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Stein J. L., Park W. D., Detke S., Lichtler A. C., Shephard E. A., Jansing R. L., Phillips I. R. Regulation of histone gene expression in HeLa S3 cells. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1107–1120. doi: 10.1101/sqb.1978.042.01.112. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Appella E., Jay G. Developmental activation of the H-2K gene is correlated with an increase in DNA methylation. Cell. 1983 Dec;35(2 Pt 1):457–465. doi: 10.1016/0092-8674(83)90179-4. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]