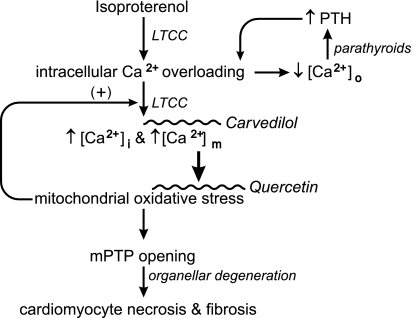
Fig. 8.
A schematic representation of the signal-transducer-effector pathway leading to cardiomyocyte necrosis and replacement fibrosis of myocardium following a single subcutaneous dose of isoproterenol given to simulate an acute hyperadrenergic stressor state. Catecholamine-induced intracellular Ca2+ overloading, expressed via increased Ca2+ entry through l-type Ca2+ channels (LTCC), leads to the rise in cytosolic [Ca2+]i and mitochondrial [Ca2+]m that contribute to an associated fall in plasma ionized [Ca2+]o. Ionized hypocalcemia provokes the Ca2+-sensing receptor of the parathyroid glands to promote the secretion of parathyroid hormone (PTH). This calcitropic hormone further contributes to intracellular Ca2+ overloading. The excessive accumulation of [Ca2+]m accounts for the induction of oxidative stress in these organelles and increased opening potential of their inner membrane mPTP. The ensuing osmotic swelling and degeneration of these organelles, coupled with the loss of ATP synthesis, perpetuates cardiomyocytes necrosis and consequent replacement fibrosis, or scarring of myocardium. Reactive oxygen species further contribute to intracellular Ca2+ entry via LTCC.