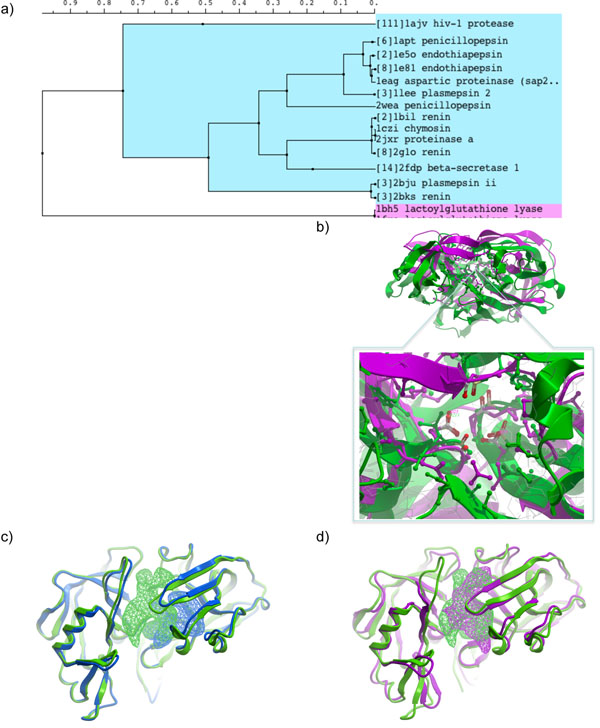
Figure 6.
(a) APF clustering sub-tree containing aspartic proteases. Branches containing only multiple structures of the same protein are collapsed and the number of structures is indicated in square brackets. (b) Superposition of HIV protease and endothiapepsin. Closeup of the binding site reveals correct superposition of the catalytic aspartic acid pair. (c,d) Comparison of binding site pockets in two renin structures (1bil, green, and 2bks, blue), (c); and in chymosin (1czi, magenta) versus renin (1bil, green), (d). Due to alternative side-chain conformations and some backbone movement, very different binding pockets are seen in the two renin structures. The pockets in the chymosin/renin pair overlay much better, which explains why in the clustering tree 1bil and 1czi are adjacent while 2bks is on a relatively remote branch. Pocket blobs were generated using icmPocketFinder[13] and visualized in ICM.