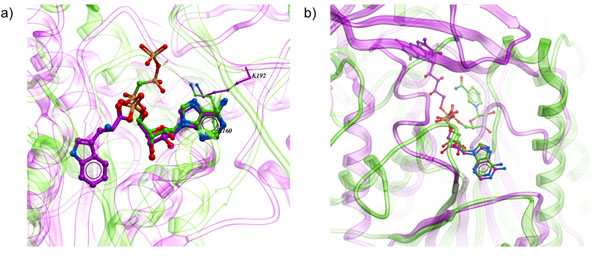
Figure 7.
Example of APF superposition of the distantly homologous binding sites: (a) tryptophanyl-tRNA synthase (1i6m, magenta) and pantothenate synthase (1n2g, green). Despite divergent functions, substrates of both enzymes contain adenosyl moiety recognized by relatively conserved motifs. Also of note is the functional mimicry of certain side-chains belonging to different segments of the structure, such as K192 in 1i6m playing the role of K160 in 1n2g, both providing a hydrogen bond to the same nitrogen in adenyl moiety. Overall sequence identity of the two enzymes is 18%. (b) NAD binding site in UDP-galactose 4-epimerase (1ek5, green) and FAD binding site in D-amino acid oxydase (1ve9, magenta). The two enzymes share similar Rossman fold sub-domains binding adenosyl moiety, while their other sub-domains are very different. Parts of well-superimposed ?-?-?-?-? structure can be seen at the bottom of the figure (transparent ribbons).