Fig. 1.
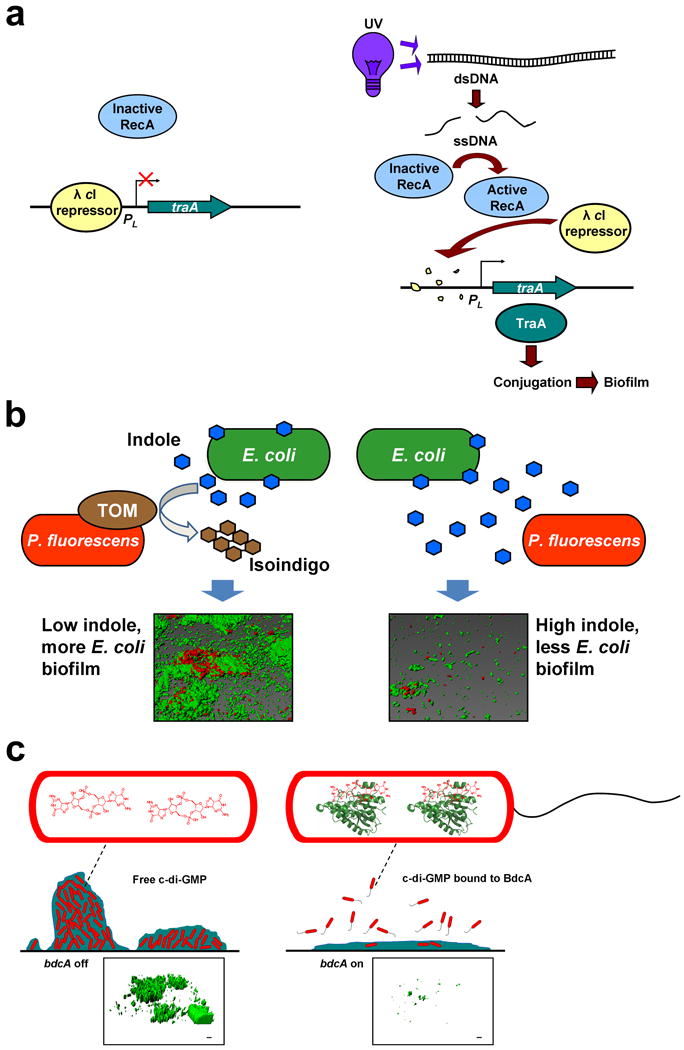
Engineered genetic circuits to control biofilm formation. (a) Genetic circuit to control E. coli biofilm formation using an environmental signal (e.g. UV light). Phage λ cI repressor silences traA transcription by binding its promoter. Application of UV light converts double-stranded DNA (dsDNA) into single-stranded DNA (ssDNA), which activates the RecA protease. Active RecA degrades λ cI, which induces traA. traA expression increases biofilm formation by enhancing conjugation. Reproduced with permission from Ref. [45]. (b) Genetic circuit to control dual-species biofilm formation using QS. Toluene o-monooxygenase (TOM) is produced by cloning tomA0A1A2A3A4A5 of Burkholderia cepacia G4 into Pseudomonas fluorescens using a constitutive promoter, which converts indole into insoluble isoindigo. Once indole is removed by the pseudomonad, indole no longer inhibits E. coli biofilm formation. Representative confocal consortial biofilm images are shown in which P. fluorescens is tagged with a red fluorescent protein and E. coli is tagged with a green fluorescent protein. Reproduced with permission from Ref. [49]. (c) Genetic circuit to control E. coli biofilm dispersal using a c-di-GMP-binding protein. Engineered BdcA variant binds c-di-GMP, which decreases the concentration of free c-di-GMP. Lower c-di-GMP concentrations lead to increased motility and reduced adhesin production, resulting in biofilm dispersal. Reproduced with permission from Ref. [63]. Representative confocal biofilm images are shown in which E. coli is tagged with a green fluorescent protein.
