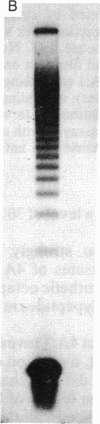
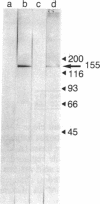
Abstract
The Plasmodium falciparum-derived antigen Pf155 contains two blocks of tandemly repeated amino acid sequences. A pair of complementary oligonucleotides, encoding the C-terminally located repeat Val-Glu-His-Asp-Ala-Glu-Glu-Asn, were synthesized. The oligonucleotides were polymerized by ligation, and the resulting multimers were cloned into an expression vector. One construct that contained four copies of the repeat was expressed in Escherichia coli. The product, a fusion protein, was soluble and produced in high amounts. It reacted in immunoblotting with a monoclonal antibody to a synthetic octapeptide (Glu-Glu-Asn-Val-Glu-His-Asp-Ala). Rabbits immunized with partially purified fusion protein, either with or without adjuvant, formed antibodies against this octapeptide. These antibodies reacted with Pf155 both in parasite extracts and when deposited in the membrane of infected erythrocytes. Furthermore, these antibodies inhibited merozoite reinvasion in vitro as efficiently as human antibodies to the octapeptide sequence in Pf155, induced by natural infection. The results suggest that products of synthetic gene constructs may be a suitable basis for an anti-merozoite vaccine.
Full text
PDF

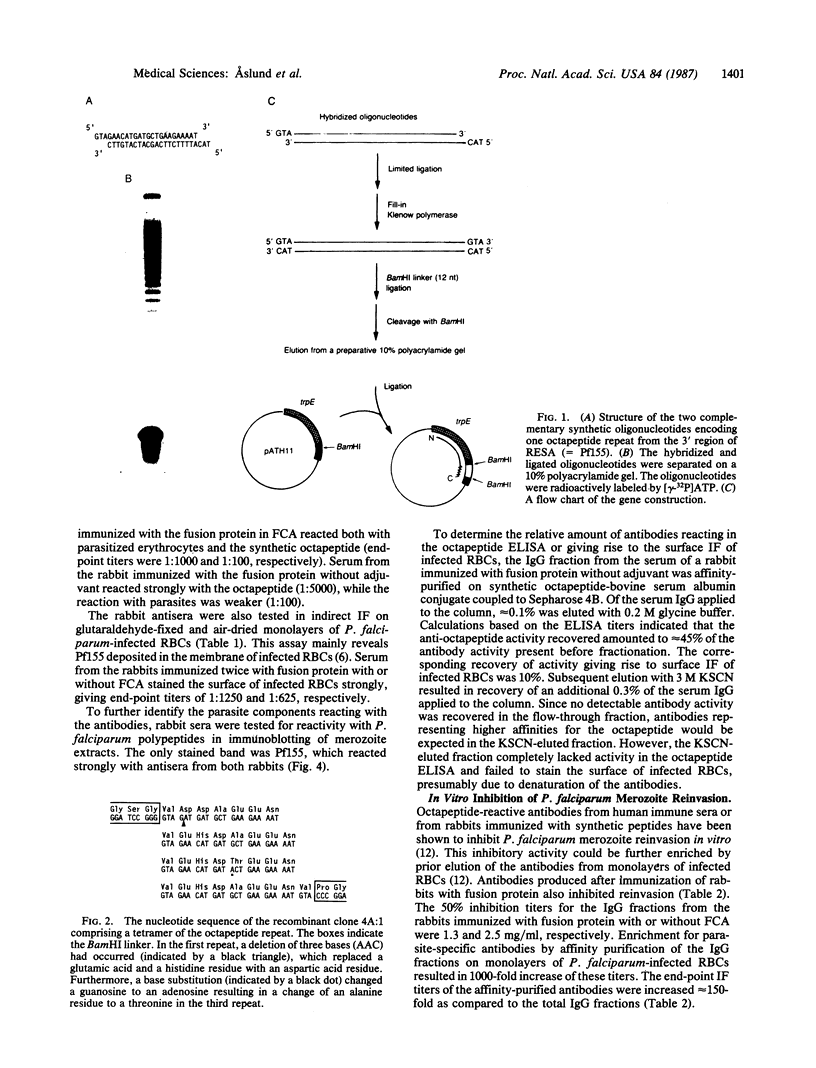
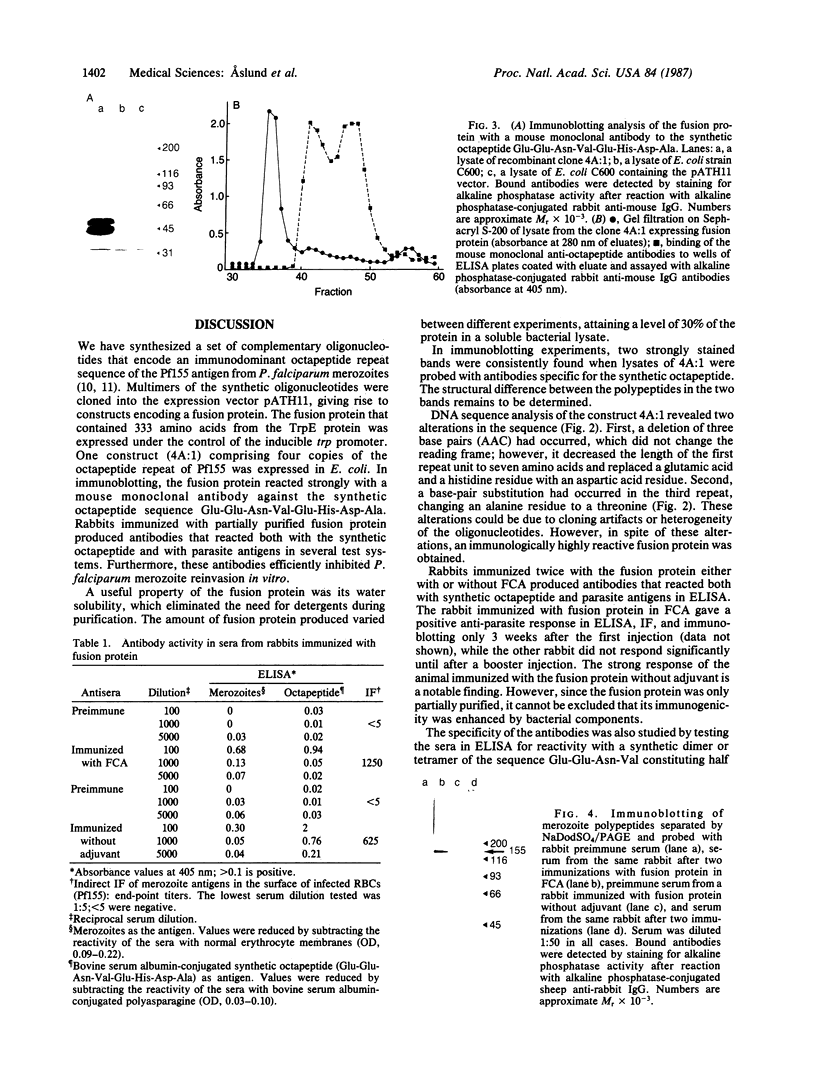

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Berzins K., Perlmann H., Wåhlin B., Carlsson J., Wahlgren M., Udomsangpetch R., Björkman A., Patarroyo M. E., Perlmann P. Rabbit and human antibodies to a repeated amino acid sequence of a Plasmodium falciparum antigen, Pf 155, react with the native protein and inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1065–1069. doi: 10.1073/pnas.83.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins K., Wahlgren M., Perlmann P. Studies on the specificity of anti-erythrocyte antibodies in the serum of patients with malaria. Clin Exp Immunol. 1983 Nov;54(2):313–318. [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Coppel R. L., Saint R. B., Favaloro J., Crewther P. E., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F., Kemp D. J. The ring-infected erythrocyte surface antigen (RESA) polypeptide of Plasmodium falciparum contains two separate blocks of tandem repeats encoding antigenic epitopes that are naturally immunogenic in man. Mol Biol Med. 1984 Jun;2(3):207–221. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazier D., Mellouk S., Beaudoin R. L., Texier B., Druilhe P., Hockmeyer W., Trosper J., Paul C., Charoenvit Y., Young J. Effect of antibodies to recombinant and synthetic peptides on P. falciparum sporozoites in vitro. Science. 1986 Jan 10;231(4734):156–159. doi: 10.1126/science.3510455. [DOI] [PubMed] [Google Scholar]
- Miller L. H., David P. H., Hadley T. J. Perspectives for malaria vaccination. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):99–115. doi: 10.1098/rstb.1984.0112. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Björkman A. Characterization of the humoral immune response in Plasmodium falciparum malaria. I. Estimation of antibodies to P. falciparum or human erythrocytes by means of microELISA. Clin Exp Immunol. 1983 Oct;54(1):127–134. [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Björkman A., Perlmann H., Berzins K., Perlmann P. Anti-Plasmodium falciparum antibodies acquired by residents in a holoendemic area of Liberia during development of clinical immunity. Am J Trop Med Hyg. 1986 Jan;35(1):22–29. doi: 10.4269/ajtmh.1986.35.22. [DOI] [PubMed] [Google Scholar]
- Wåhlin B., Wahlgren M., Perlmann H., Berzins K., Björkman A., Patarroyo M. E., Perlmann P. Human antibodies to a Mr 155,000 Plasmodium falciparum antigen efficiently inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7912–7916. doi: 10.1073/pnas.81.24.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]