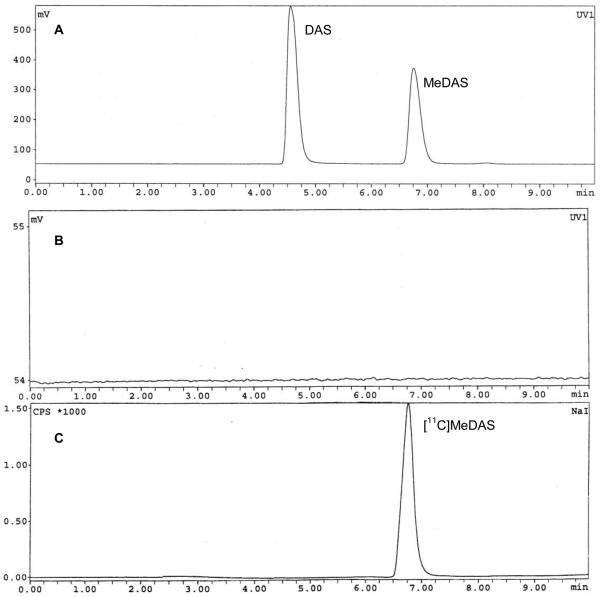
Figure 1.
Quality control HPLC figures for both UV and radioactive peaks. A: Coinjection of precursor DAS and non-labeled cold standard of MeDAS, the rentention times were 4.57 min and 6.77 min, respectively. B: Quality control by analytical radio-HPLC showed the quantity of the precursor DAS in the final product is negligible: no DAS residue was detected after HPLC purification. C: Quality control by analytical radio-HPLC showed a radiochemical purity of the final product is greater than 95%, the retention time of [11C[MeDAS was 6.77 min, which was consistent with the non labeled cold standard MeDAS.