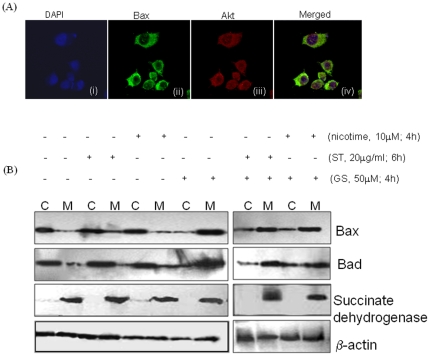
Figure 5. (A) AKT is co-localized with Bax in cytoplasm.
SCC4 cells (5×103) were plated on coverslips and incubated with a mouse antibody against human Bax and a rabbit antibody against human AKT antibodies. Alexa flour®594 -conjugated anti-rabbit (red) and Fluorescein isothiocyanate-conjugated (green) anti-mouse secondary antibodies were used to visualize Akt (red) and Bax (green) localization patterns using a fluorescent microscope. Panel (i) DAPI stained nuclei in blue color; (ii) cytoplasmic expression of Bax; (iii) cytoplasmic expression of Akt protein; and (iv) merged photomicrograph (ii) and (iii) showing co-localization of Bax and Akt. (B) Phosphorylation of Bax at (Ser-184) and Bad (Ser-136) results in retention of Bax and Bad in cytosol. SCC4 cells were kept untreated or treated with 50 µM GS for 4 h, ST (20 µg/ml) for 6 h, 10 µM nicotine for 4 h. SCC4 cells were pre-treated with 50 µM GS for 4 h, followed by ST for 6 h or with nicotine for 4 h. Cytoplasmic (C) and Mitochondrial (M) extracts were prepared as described in materials and methods and separated on 10% SDS-PAGE. Proteins were then electro-transferred on PVDF membrane followed by blocking with 5% non-fat milk overnight. Blots were incubated with specific antibodies against Bax and Bad. Protein expression was determined using enhanced chemiluminescence method. Purity of the subcellular fractions obtained was determined using western blot for mitochondrial protein, succinate dehydrogenase. β-actin was used as a loading control.