Abstract
There is increasing evidence that the aryl hydrocarbon receptor (AHR) plays a role in tumor progression through numerous mechanisms. We have previously shown that, in certain cancer cell lines that are typically non-responsive to cytokine-mediated IL6 induction, activation of the AHR with the agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin derepresses the IL6 promoter and allows for synergistic induction following IL1β treatment. The mechanism by which this occurs involves liganded AHR binding upstream from the transcription start site and dismissing HDAC-containing corepressor complexes, giving rise to a promoter structure that is more amenable to NF-κB activation. This fact, combined with observations of multiple endogenously-produced chemicals activating the AHR, led us to study its role in basal expression among high cytokine-producing cancer cell lines. The current study provides evidence that several head and neck squamous cell carcinoma cell lines have a level of constitutively bound AHR at the IL6 promoter, allowing for higher basal and readily inducible IL6 transcription. Treatment of these cell lines with an AHR antagonist led to dismissal of the AHR from the IL6 promoter and recruitment of corepressor complexes, thus diminishing cytokine expression. Head and neck squamous cell carcinoma is typically a high cytokine-producing tumor type, with IL6 expression levels correlating with disease aggressiveness. For this reason, AHR antagonist treatment could represent a novel adjuvant therapy for patients, lowering pro-growth and anti-apoptotic signaling with minimal systemic side effects.
Keywords: AHR, aryl hydrocarbon receptor, IL6, cytokines, antagonist
INTRODUCTION
The aryl hydrocarbon receptor (AHR) has been historically examined as a mediator of response to xenobiotic exposure, leading to subsequent metabolism of the compounds. A ligand-activated transcription factor of the basic helix-loop-helix, Per-Arnt-Sim class of proteins, research has begun to show that the AHR plays numerous physiological roles outside of its xenobiotic niche and does so through various molecular mechanisms. The AHR-mediated signaling pathway has been documented extensively, and recent reviews highlight the array of modes through which the AHR produces its effects (1). Prior to activation, the AHR resides largely in the cytoplasm, in a core complex with a 90 kDa heat shock protein dimer (hsp90) and the X-associated protein 2 (XAP2). Following activation by agonist binding, the receptor translocates to the nucleus, where it releases its chaperone proteins and dimerizes with its partner protein, the aryl hydrocarbon receptor nuclear translocator (ARNT). The AHR binds a variety of xenobiotics including polycyclic aromatic hydrocarbons (PAHs) such as benzo[a]pyrene (B[a]P) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). PAHs are common environmental pollutants resulting from car exhaust, manufacturing, iron foundries, and cigarette smoke, in addition to other sources. The xenobiotic role of the AHR has typically been studied in reference to its ligand-mediated binding to dioxin response elements (DREs) in the promoters of cytochrome P4501A genes, which express enzymes that act in phase I drug metabolism. Research into the disparate endogenous activities of the AHR has shown that it plays a role in Th17 immune cell differentiation, regulation of acute phase response genes, antiestrogenic activities, and modulation of NF-κB protein activity (2–5).
Several mechanisms have been documented by which the AHR can affect gene regulation, as outlined in the review by Beischlag, et al (1). The prototypical AHR activation pathway involves ligand activation, heterodimerization with ARNT and binding to DRE sequences in the promoter of a target gene to regulate transcription. Multiple instances of protein-protein interactions have been demonstrated, including AHR interactions with ERα (6), RELB (7), glucocorticoid receptor (8), and β-catenin (9). This last interaction is due to the AHR acting as an E3 ubiquitin ligase and inducing turnover of β-catenin. Additionally, ligand binding by the AHR has been shown to affect other cellular processes through mechanisms unknown at this time, such as the ability to repress acute phase response genes in the absence of DRE binding (3).
We have previously shown that ligand-activated AHR plays a role in the synergistic induction of IL6 following IL1β cotreatment in MCF-7 breast cancer cells (10, 11). In these cells, the presence of an AHR ligand or an inflammatory signal (e.g., IL1β) alone leads to only a modest level of IL6 induction. The mechanism by which the presence of AHR at the IL6 promoter mediates IL6 induction in what is typically an unresponsive cell line centers on the activated AHR/ARNT heterodimer binding to imperfect DREs upstream from the IL6 transcription start site and displacing corepressor complexes. This in turn allows for IL1β-mediated induction of IL6 through recruitment of NF-κB family members to the promoter. The presence of the HDAC1-containing corepressor complex at the IL6 promoter is at least partially responsible for preventing basal expression, and perhaps plays a role in the weakly metastatic phenotype of MCF-7 cells. Comparatively, aggressive cell lines often display high constitutive cytokine expression, as well as highly invasive and metastatic phenotypes. Following elucidation of the mechanism by which the AHR mediates the de-repression of the IL6 promoter in some cell lines, our research turned to whether the AHR plays a role in constitutive IL6 expression in a variety of tumor cell lines.
IL6 induction is most commonly seen in acute phase response signaling. Cancer cells have been shown to express IL6 in certain situations, often accompanied by phenotypic changes. Prostate cancer cells have been shown to have increased anti-apoptotic properties, and prostate and breast cancer cells have both been shown to have increased chemo-resistance in conjunction with higher IL6 production (12, 13). Likewise, breast cancer cells have been shown to have decreased adhesive properties and greater migratory ability, along with increased proliferation, following an increase in IL6 production (14–17).
Squamous cell carcinoma of the head and neck (HNSCC) is an umbrella term that covers solid tumors of the larynx, pharynx, oral cavity, tongue, and nasal passages. Squamous cell carcinoma of the head and neck (HNSCC) has been tied to high cytokine expression both in vitro and in human patients (18–20). Expression of IL6 in HNSCC is associated with increased disease invasiveness, as well as patient prognosis and recurrence rates (21). The results of the current study point to a level of constitutively active AHR in numerous HNSCC cell lines, which leads to a direct effect on IL6 mRNA and protein expression. An AHR antagonist is able to significantly attenuate IL6 expression by reducing the level of AHR occupancy at the IL6 promoter, and thus allow for re-occupancy by the corepressor complex observed previously (11). In this manner, treatment of HNSCC tumors with an AHR antagonist could represent a well-tolerated method by which pro-growth and metastasis signaling could be reduced prior to typical chemotherapy and radiation therapy.
MATERIALS AND METHODS
Chemicals
6, 2′, 4′-trimethoxyflavone (TMF) was purchased from Indofine Chemical Company 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was kindly provided by Dr. Steve Safe, Texas A&M University
Cell culture
All head and neck squamous cell carcinoma [HNSCC] lines (HN4, HN13, HN15, HN30, HN2095) were maintained at 37°C, 5% CO2 in a high glucose 1:1 DMEM:F12 (Sigma), supplemented with 10% fetal bovine serum (FBS; Hyclone Labs.), 1,000 units/ml penicillin, and 0.1 mg/ml streptomycin (Sigma). HN4, HN15, and HN2095 cells were obtained from Susan Mallery, following their purchase from ATCC (22, 23). HN13 and HN30 cells were obtained from J. Silvio Gutkind and were first developed and characterized by Yeudall, et al (24). BEAS-2B cells were purchased from ATCC and maintained in supplemented BEBM media with 1,000 units/ml penicillin, and 0.1 mg/ml streptomycin (Sigma).
Gene expression
All HNSCC cell lines were serum-starved 18 h before treatment. Treatment of cells was performed by diluting compounds to the desired working concentration in serum-free media supplemented with 5 mg/ml bovine serum albumin (BSA). Total RNA was extracted from the cells using TRI reagent (Sigma) as specified by the manufacturer. The ABI high-capacity cDNA archive kit (Applied Biosystems) was used to prepare cDNA from isolated RNA. mRNA expression for all samples was measured by quantitative real-time PCR using the Quanta SYBR Green kit on an iCycler DNA engine equipped with the MyiQ single color real-time PCR detection system (Bio-Rad). Expressed quantities of mRNA were normalized to GAPDH or L13A mRNA levels and plotted using GraphPad Prism 4.0 (GraphPad Software). Histograms are plotted as mean values of three biological replicates, error bars represent the standard deviation of replicates. Real time primers used are shown in supplemental methods. Statistical significance was calculated using the student’s T test, one-way ANOVA, and two-way ANOVA, as appropriate for the number of values and comparisons made.
Immunoblotting
Whole cell extracts were prepared by lysing cells in 1× radioimmunoprecipitation assay buffer [RIPA; 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 140 mM NaCl, 1% Triton X-100, 0.1% Na-deoxycholate, 0.1% SDS] supplemented with 1% NP40, 300 mM NaCl, and protease inhibitor cocktail (Sigma). Homogenates were centrifuged at 21,000×g for 30 min at 4° C, and the soluble fraction was collected as whole cell extract. Protein concentrations were determined using the detergent compatible DC protein assay kit (Bio-Rad). Protein samples were resolved by Tricine SDS-PAGE and transferred to PVDF membrane. Primary antibodies used to detect specific proteins are shown in supplemental methods and were visualized using biotin-conjugated secondary antibodies (Jackson Immunoresearch) in conjunction with 125I streptavidin (Amersham). Quantification of protein levels was conducted in triplicate by removal of 125I labeled protein bands and analysis of radioactivity via gamma counter.
Chromatin immunoprecipitation assays
HNSCC cell lines were grown to approximately 90% confluency in 150 cm2 dishes and serum-starved 18 h before treatment. Cells were treated in serum-free media supplemented with 5 mg/ml BSA by diluting compounds to the desired working concentrations. Following treatment, cells were washed once with warm PBS and chromatin complexes were chemically crosslinked using a 1% formaldehyde/PBS solution (final concentration) for 10 min at room temperature. Crosslinking was stopped by addition of glycine solution to a final concentration of 0.125 M, cells were then washed twice with ice cold PBS, scraped, and collected in 2 ml of harvest buffer (100 mM Tris, pH 8.3, 10 mM dithiothreitol). Cells were centrifuged, washed in ice cold PBS, and resuspended in 600 μl of lysis buffer (1% SDS, 50 mM Tris-HCl, pH 8.1, 10 mM EDTA). Chromatin was sheared with the Bioruptor water bath sonicator (Diagenode, Sparta, NJ) to an average size of 500 bp - 1 kb. Complexes were precleared with protein A agarose (Pierce) and incubated overnight with specific antibodies, listed in supplemental methods. Immunoadsorbed complexes were captured on protein A agarose and washed once with TE8 (10 mM Tris-HCl (pH 8.0) and 0.5 M EDTA). Agarose-bound complexes were then resuspended in TE8, layered on top of a sucrose solution (1 M sucrose, 200 mM NaCl, 1% NP40) and centrifuged for 3 min. Agarose-bound complexes were then washed once with 0.5× RIPA buffer, followed by four washes with TE8. Samples were eluted off the agarose using 200 μl elution buffer (100 mM NaHCO3, 1% SDS), and crosslinks were reversed at 65° C overnight. Eluted DNA was isolated, washed, and concentrated using the ChIP DNA Clean & Concentrator Kit (ZYMO Research). Immunoadsorbed DNA was analyzed by PCR with primers listed in supplemental methods. Results shown are representative of multiple experiments.
Gene silencing
Specific protein levels were decreased using the Dharmacon small interfering RNA (siRNA) [control oligo D-001810-0X, AHR oligo J-004990-07, BRG1 (SMARCA4) oligo D-010431-01]. Electroporation/nucleofection was performed using the Amaxa nucleofection system essentially as described in manufacturer protocols. Briefly, cells were washed and suspended at a concentration of 2.0 × 106 per 100 μL of nucleofection solution. Control or targeted siRNA was added to the sample for a final concentration of 1.5 μmol/L. Samples were electroporated using manufacturer’s MCF-7 high efficiency program and plated into six-well dishes in complete media.
ELISA assay
To quantify IL6 protein expression, media were collected from cell lines treated for 24 h with vehicle or 10 μM TMF and frozen at −80°C until analyzed. Briefly, 96-well optical dishes were prebound with 50 μL of 2 μg/ml anti-human IL6 monoclonal antibody (R&D Systems) in 0.1 M NaHCO3 overnight at 4°C. Plates were washed extensively with PBS plus 0.05% Tween 20 (PBST) and then blocked with 1% Block Ace diluted in nanopure water for at least 2 h. Plates were washed extensively with PBST. Samples were added (100 μL/well) in duplicate and incubated overnight at 4°C. Recombinant human IL6 (10,000-10 pg/ml; R&D Systems, rh IL6) diluted in serum-free culture media was used as a standard. Plates were washed with PBST and incubated with 100 μL of 0.2 μg/ml biotinylated goat anti-human IL6 antibody (R&D Systems) for 2 h. Plates were washed extensively with PBST and incubated with 100 μL of 1 μg/ml streptavidin (Pierce) diluted in PBS for 30 min at room temperature. Plates were washed extensively with PBST, followed by a final incubation with 100 μL of a 0.3 mg/ml 2,2-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) and 100 mM citric acid solution (pH 4.35) plus 0.03% H2O2 in the dark. Colorimetric assay was performed 60 min later by reading absorbance at 405 nm on a Spectracount spectrophotometer, and data were analyzed using I-Smart software (Perkin-Elmer).
RESULTS
Both benign cells of the human airway and malignant cells of the oral cavity have readily inducible IL6
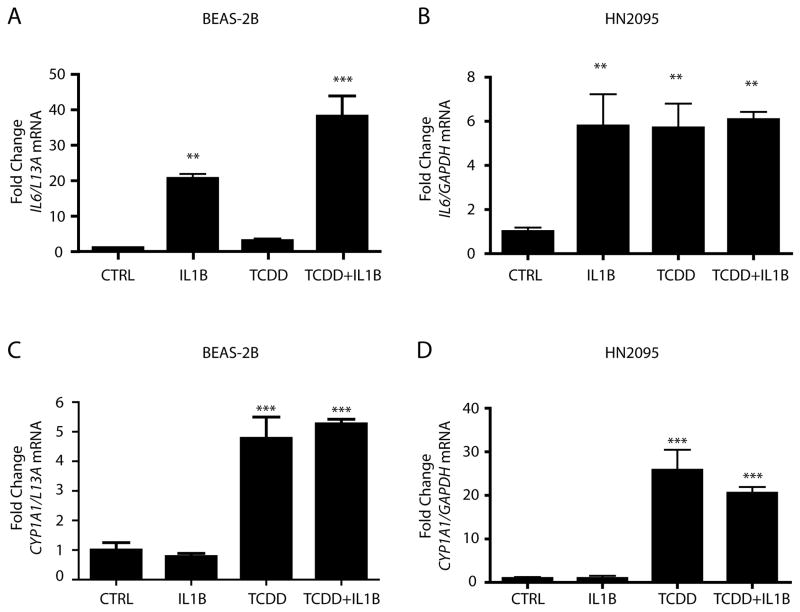
Our previous research has explored the manner in which the activated AHR primes MCF-7 human breast cancer cells for IL1β-mediated IL6 induction. MCF-7 cells are typically seen as unresponsive to cytokine-mediated IL6 induction and exhibit relatively low levels of basal and induced IL6 expression when compared to more aggressive breast cancer lines, such as MDA-MB231 cells (25). The molecular mechanism by which the AHR mediates synergistic IL6 expression in MCF-7 cells centers on ligand-bound AHR occupying imperfect DREs in the IL6 promoter and dismissing HDACs, which then allows for full IL1β-mediated recruitment of NF-κB family members and leads to a transcriptional increase (11). However, not all cell lines display constitutively repressed IL6 promoters, as evidenced by the benign bronchial epithelial cell line BEAS-2B, and the human HNSCC cell line 2095 (HN2095) (Fig 1A). While BEAS-2B cells do undergo a statistical synergy with TCDD and IL1β cotreatment, the ability of IL1β alone to induce significant IL6 expression points to a promoter structure that is more readily accessible to inflammatory signals in its native state than that observed in MCF-7 cells. IL6 in BEAS-2B cells is only slightly more inducible following AHR activation. HN2095 cells show higher basal IL6 expression relative to maximal induction levels, and achieve full induction following IL1β treatment alone. Interestingly, TCDD treatment alone also mediates IL6 induction (Fig 1B). This event was examined in two other HNSCC cell lines, HN13 and HN30, both of which were also shown to have significant IL6 induction following TCDD treatment (Supp Fig 1). Activation of the AHR following TCDD treatment leads to significant increases in CYP1A1 transcription in both cell lines (Figs 1C, 1D), illustrating that the AHR exhibits considerable transactivation potential. We have previously demonstrated that AHR-mediated IL6 synergy in MCF-7 cells is dependent upon activated AHR/ARNT complexes binding to imperfect DREs in the IL6 promoter. The lack of significant basal CYP1A1 transcription in both BEAS-2B and HN2095 cells suggests that the presence of substantial levels of endogenous AHR ligands that behave as agonists is unlikely.
Fig 1. Both nonmalignant and malignant cells of the head and neck display readily inducible IL6.
Serum starved BEAS-2B (A,C) and HN2095 (B,D) cells were treated for 2 h with vehicle, 10 ng/ml IL1β, 1 nM TCDD, or TCDD+IL1β. Total RNA was then isolated, cDNA prepared, and relative IL6 (A,B) and CYP1A1 (C,D) mRNA levels were determined by quantitative real-time PCR.
AHR is required for basal and induced IL6 in HN2095 cells
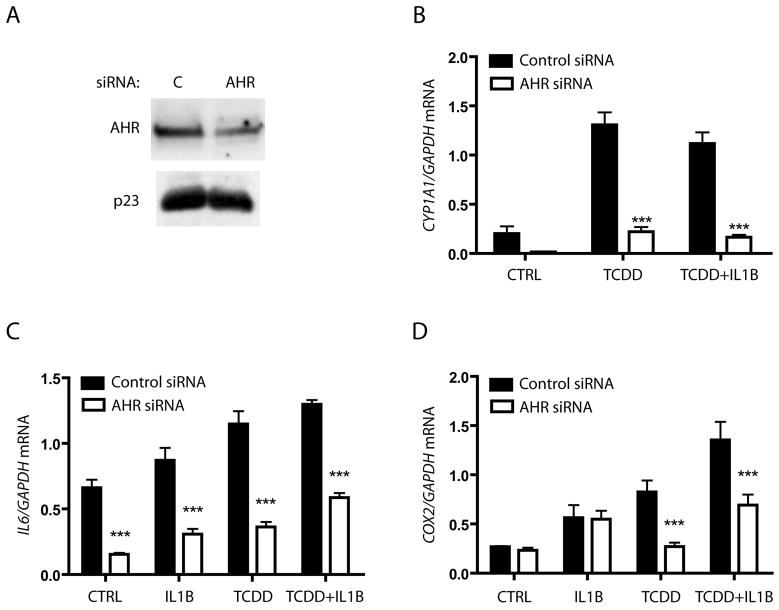
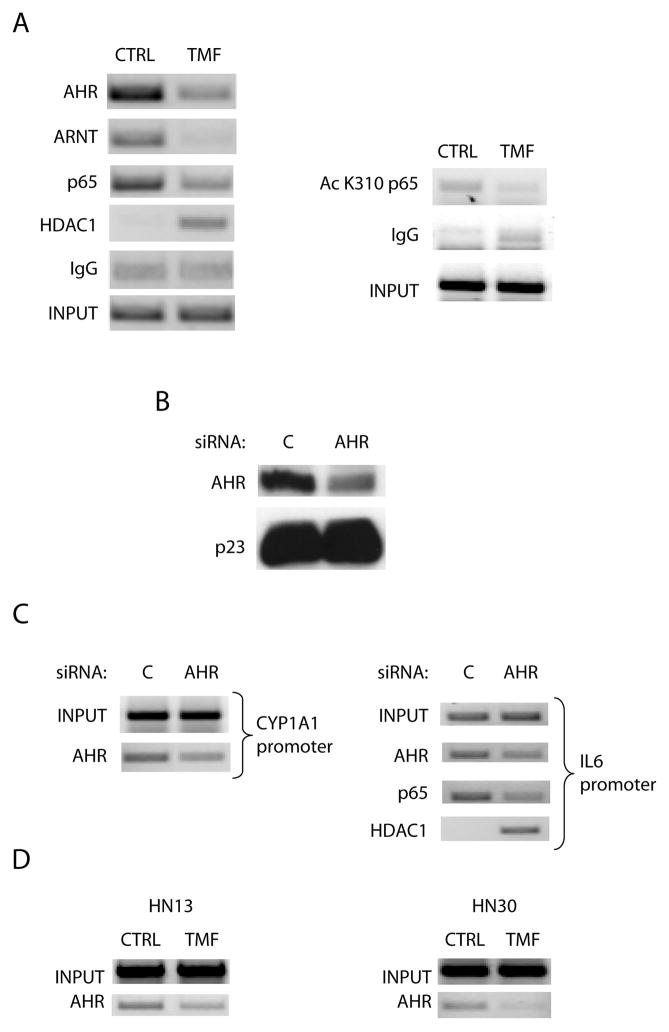
The synergistic induction of IL6 observed in MCF-7 cells following cotreatment with IL1β and an AHR ligand was clearly found to be an AHR/ARNT mediated effect (11). However, the fact that HN2095 cells did not show constitutively high CYP1A1 expression called into question whether a basal level of AHR was constitutively occupying the IL6 promoter, leading to elevated basal IL6 expression. Furthermore, it is also possible that the IL6 promoter was accessible to IL1β-mediated induction in an AHR-independent manner. To differentiate between these possibilities, HN2095 cells were transfected with AHR-targeting siRNA oligonucleotides, which resulted in a greater than 50% decrease in AHR protein levels; a representative protein blot is shown in figure 2A. AHR knockdown was sufficient to block the low level of constitutive expression and significantly reduce TCDD-mediated CYP1A1 activity (Fig 2B). Incomplete knockdown of AHR protein prevented full reduction of IL6 levels, but led to significant reductions in constitutive levels, as well as cytokine and AHR ligand induced levels (Fig 2C). COX2 is known to be an inflammatory response gene that is up-regulated by both IL1β and TCDD, though the two have not been linked to a common induction pathway. AHR knockdown in HN2095 cells resulted in loss of TCDD-induced COX2 induction, but failed to change basal and IL1β-induced levels (Fig 2D). Thus, the AHR appears to play a direct role in IL6 transcription, while only mediating TCDD-induced COX2 expression.
Fig 2. AHR plays a role in both basal and induced IL6 expression in HNSCC.
HN2095 cells were electroporated with control or AHR siRNA, plated for 24 h, and serum starved for 18 h. A) Whole cell extracts were prepared and expression levels of AHR and p23 (control) were assessed by immunoblot. B–D) Cells were electroporated as in A, plated into 6 well dishes, then serum starved before being treated for 2 h with vehicle, 10 ng/ml IL1β, 1 nM TCDD, or TCDD+ IL1β. Total RNA was isolated, cDNA prepared, and relative CYP1A1, IL6, and COX2 mRNA levels were determined by quantitative real-time PCR.
An AHR antagonist can reduce basal and induced IL6 mRNA and protein levels in multiple HNSCC cell lines
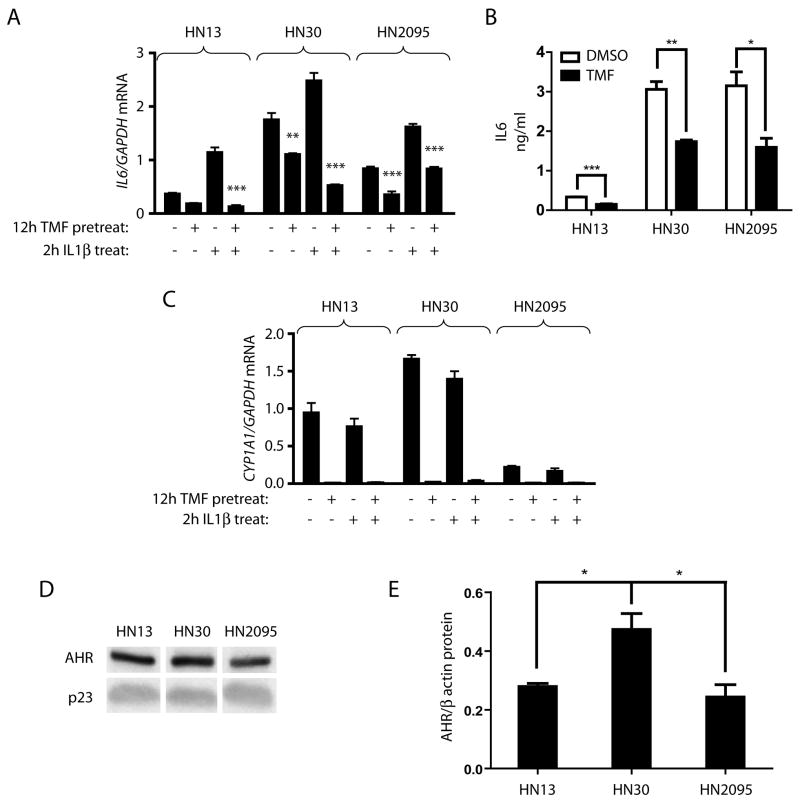
IL6 has been shown to lead to pro-growth signaling in various cancer cell lines, and its up-regulation has also been noted in numerous head and neck squamous cell carcinoma lines. The heterogeneity of the cell population contained under the HNSCC classification led us to investigate whether different HNSCC cell lines showed the same AHR involvement in IL6 transcriptional regulation as observed in HN2095. This hypothesis was evaluated using TMF, an AHR antagonist that inhibits receptor activated events in the presence of agonist activity (26). Treating three HNSCC cell lines for 12 h with 10 μM TMF significantly lowered basal IL6 gene expression in two of the lines; HN30 and HN2095. TMF pretreatment prior to 2 h IL1β treatment led to a significant decrease in induced IL6 levels in all three HNSCC lines tested (Fig 3A). The same effect was also seen with HN4 and HN15 cell lines (Supp Fig 1A), but HN13, HN30 and HN2095 were selected for further study as they appeared to represent varying levels of basal and inducible IL6 expression. HN13, HN30 and HN2095 cells further showed significantly lower basal IL6 protein levels following TMF treatment (Fig 3B). The variability of IL6 expression between lines is evidenced by the fact that HN30 has two-fold higher basal IL6 expression than HN2095 which, in turn, has greater than two-fold higher basal expression than HN13. HN30 also shows basal CYP1A1 levels significantly higher than the other cell lines. Variable expression levels of the AHR protein among the three lines could account for some of the difference between basal expression of AHR target genes. Untreated HN30 cells have a significantly higher level of AHR protein expression as compared to the other lines (Fig 3C, 3D, Supp Fig 2B).
Fig 3. AHR antagonist treatment reduces IL6 mRNA and protein in numerous AHR expressing HNSCC cell lines.
Serum starved HN13, HN30, and HN2095 cells were treated for 12 h (A,C) or 24 h (B) with vehicle or 10 μM TMF. A) Following 12 h pretreatment, cells were treated with vehicle or 10 ng/ml IL1β for 2 h. Total RNA was then isolated, cDNA prepared, and relative IL6 mRNA levels were determined by quantitative real-time PCR. Significance testing indicates that TMF pretreatment decreases basal and IL1β-induced IL6 expression. B) Media was collected following 24 h treatment and subjected to ELISA. C) mRNA from 12 h TMF treatments in (A) were assessed for CYP1A1 expression. D,E) HN13, HN30 and HN2095 cells were plated and serum starved, after which whole cell extracts were made. D) Representative western blot showing endogenous AHR levels. E) Endogenous AHR levels were quantified and normalized to p23 control. Samples were in triplicate.
AHR involvement in inflammatory signaling is both gene and cell line specific
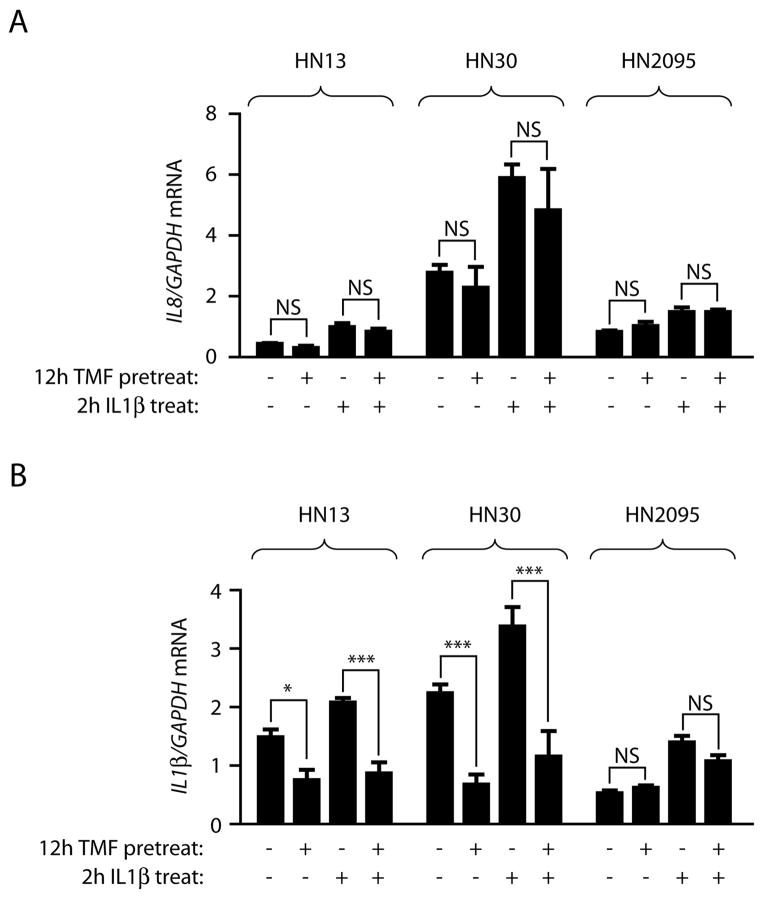
The finding that siRNA-mediated knockdown of AHR protein resulted in a loss of TCDD-induced COX2 expression but not IL1β-induced COX2 in HN2095 cells (Fig 2D) led to the question of whether TMF treatment would similarly show a targeted role in inflammatory mediation. Treatment of HN13, HN30 and HN2095 cells for 12 h with 10 μM TMF did not affect basal IL8 expression levels or IL1β-mediated IL8 induction across the three cell lines (Fig 4A). However, in examining IL1β transcription levels, TMF treatment leads to a significant repression in both HN13 and HN30 cell lines, but has no effect on HN2095 cells (Fig 4B). Taken together, these data argue that the TMF concentrations used in these experiments do not result in a generalized pro- or anti-inflammatory effect. However, the role played by the AHR in IL1β expression remains unclear, and is cell line dependent.
Fig 4. The role played by the AHR in inflammatory signaling is both cell line- and gene-specific.
HN13, HN30 and HN2095 cells serum starved and pretreated for 12 h with vehicle or 10 μM TMF prior to treatment with vehicle or 10 ng/ml IL1β for 2 h. Total RNA was then isolated, cDNA prepared, and relative mRNA levels determined by quantitative real-time PCR.
IL6 promoter analysis after TMF treatment reveals coactivator/corepressor switching
The mechanism by which activated AHR leads to synergistic IL6 production following IL1β treatment in MCF-7 cells was demonstrated to involve receptor displacement of HDAC1-containing corepressor complexes, allowing for increased recruitment and full activation of NF-κB transcriptional activators (11). The question of whether constitutive AHR occupancy of the IL6 promoter in HNSCC cell lines would lead to the same outcome was determined by ChIP assays. We have shown that HN2095 cells treated for 12 h with 10 μM TMF exhibit a significant decrease in basal AHR occupancy at the CYP1A1 promoter (26). This same treatment leads to a significant decrease in AHR and ARNT presence upstream from the transcription start site in the IL6 gene. This dismissal of AHR coincided with decreased p65 occupancy and increased HDAC1 occupancy, as would be expected from our previous studies (11). There was also a decrease in acetylated p65, a hallmark of optimal NF-κB activation (Fig 5A). siRNA-mediated repression of AHR protein expression in HN2095 cells was carried out (Fig 5B), and control or AHR ablated cells were then similarly analyzed by ChIP. AHR knockdown is shown to result in effects similar to TMF treatment; namely, AHR occupancy is shown to be decreased at the CYP1A1 promoter, and protein loss from the IL6 promoter correlates with loss of p65 and increase in HDAC1 presence (Fig 5C). Just as HN13 and HN30 show similar AHR-mediated IL6 expression, ChIP analysis of the IL6 promoter in both cell lines following TMF treatment shows evidence of a loss of basal AHR presence. Interestingly, HN30 cells, having significantly higher AHR protein levels and CYP1A1 activity, required 36 h of TMF treatment for discernible AHR dismissal from the IL6 promoter, compared to the 12 h needed in HN13 and HN2095 cells (Fig 5D). ChIP analysis of the IL6 promoter in HN2095 cells provided evidence that the presence of the AHR correlates with increased transcriptional potential.
Fig 5. Reduction of AHR occupancy at the IL6 promoter leads to a shift from coactivators to corepressors.
A) ChIP analyses of the IL6 promoter in HN2095 cells following 12 h treatment with vehicle or 10 μM TMF. B) HN2095 cells were electroporated with control or AHR targeted siRNA oligonucleotides, plated for 24 h and serum starved for 24 h. Whole cell extracts were prepared, and protein levels of AHR and p23 (control) were assessed by immunoblot. C) HN2095 cells were electroporated and serum starved for 12 h, then treated for 12 h with vehicle or 10 μM TMF. ChIP analyses of the CYP1A1 and IL6 promoters were then carried out for the specified proteins. D) Serum starved HN13 and HN30 cells were treated with 10 μM TMF for 12 h and 36 h, respectively, followed by ChIP analysis of the IL6 promoter.
AHR presence at the IL6 promoter influences IL6 expression through mediation of BRG1 activity
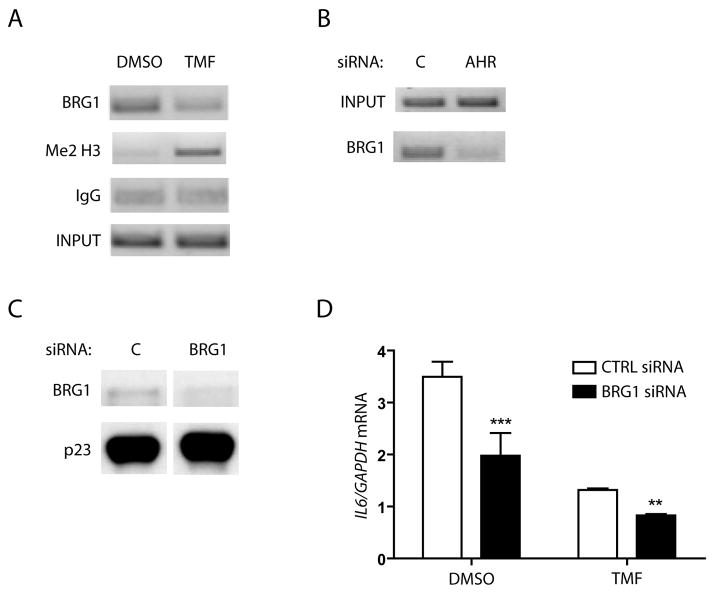
It has previously been shown that AHR ligand-induced CYP1A1 transcription relies on the AHR/ARNT heterodimer and Brahma-related gene-1 (BRG1, SMARCA4) binding in the CYP1A1 enhancer region (27, 28). BRG1 is one of two main catalytic subunits that drive the mammalian SWI/SNF complex as the ATP engine. Human SWI/SNF complexes have been shown to interact with various nuclear receptors and mediate their recruitment to gene promoters. These complexes subsequently act by changing histone methylation and influencing other post-translational modifications. Thus, BRG1 plays an integral role in regulating the chromatin structure in the promoter regions of a variety of genes (reviewed in 29). The presence of BRG1 at the IL6 promoter was assessed within the same ChIP experiments presented in figure 5. In addition to lowering basal AHR presence at the IL6 promoter, 12 h TMF treatment of HN2095 cells reduces BRG1 presence in the same region (Fig 6A). Concurrently, TMF treatment leads to a significant increase in dimethylated histone H3 on lysine 4 (Fig 6A). Studies examining the promoter assembly of the CIITA pIV gene following IFNγ stimulation have revealed that a lack of BRG1 correlates with an increase in this same histone modification and promoter repression, due to a closed chromatin conformation (30). Similarly, siRNA-mediated ablation of AHR protein expression followed by ChIP analysis revealed a parallel loss of BRG1 at the IL6 promoter (Fig 6B). In addition, transfection of HN2095 cells with BRG1-targeted siRNA oligonucleotides resulted in a substantial reduction in BRG1 expression (Fig 6C). This decrease in BRG1 protein led to a significant reduction in basal IL6 transcription, which was further reduced following TMF treatment, when compared to control siRNA transfections (Fig 6D).
Fig 6. AHR plays a role in BRG1 occupancy and modification of the IL6 promoter.
A) ChIP analysis from figure 5A of the IL6 promoter in HN2095 cells for BRG1 protein following 12 h TMF treatment. B) AHR siRNA transfection and ChIP analysis of the IL6 promoter in HN2095 cells from figures 5B,C for BRG1 protein C) HN2095 cells were electroporated with control or BRG1 siRNA, plated for 24 h, serum starved for 18 h, and whole cell extracts were then prepared. Levels of BRG1 and p23 (control) expression were assessed by immunoblot. D) HN2095 cells were electroporated, plated into 6 well dishes, then serum starved before being treated for 12 h with vehicle or 10 μM TMF. Total RNA was isolated, cDNA prepared, and relative IL6 mRNA levels were determined by quantitative real-time PCR.
DISCUSSION
It has been established that the AHR is involved in numerous physiological processes aside from its traditional role as a xenobiotic receptor. AHR activation has been shown to play an influential role in multiple inflammatory signaling pathways, macrophage function, malignant cellular phenotypes, and even cancer patient prognosis, with variation in effects seen between cell and tumor types (31–34). Our previous research has shown that activation of the AHR with an exogenous ligand derepresses the IL6 promoter in MCF-7 cells, leading to synergistic IL1β-mediated IL6 induction in what is normally an unresponsive cell line (10, 11). The determination of the mechanism, in conjunction with the knowledge that endogenous ligands for the AHR can be produced in cellular environments (35–38) led us to question whether there could be instances of high basal levels of activated AHR mediating constitutive or inducible IL6 expression.
A survey of the literature revealed that head and neck squamous cell carcinomas often exhibit elevated IL6 production. This finding led us to investigate malignant cell lines from the upper airway and related tissues. Tumors of the larynx and pharynx fall under the umbrella of squamous cell carcinoma of the head and neck (HNSCC), which also includes the oral cavity and other loco-regional tissues. Initial results showed that the commonly used HN2095 cell line had even more readily inducible IL6 expression, with IL1β treatment alone being enough for maximal induction. Both basal and induced IL6 expression have some measure of AHR dependency, thereby pointing to a level of constitutive receptor activation in HNSCC, but one which is not sufficient or competent to efficiently drive target gene expression (i.e., CYP1A1).
HNSCC cell lines have been shown to experience an autocrine loop of pro-growth and anti-apoptotic signaling, at least partly due to STAT3 activation (39). A positive feedback loop based upon IL6 autocrine signaling would presumably add to this STAT3 activation effect. Constitutively active STAT3 has likewise been seen as a hallmark of HNSCC, and is frequently considered a potential target for therapeutic intervention. STAT3-mediating therapies have, in fact, been shown to increase apoptosis and cell cycle arrest, decrease invasion, and sensitize HNSCC cell lines to chemotherapy treatment (40–43). However, systemic treatment with a compound to fully inhibit STAT3 activation or even decrease the endogenous STAT3 levels would likely induce high grade cellular side effects in a patient. When possible, HNSCC is generally treated with a combination of surgical resection via radical neck dissection followed by adjuvant chemotherapy and/or radiation therapy (44). Any pretreatment that results in systemic side effects would likely limit later treatment options, including length and dose of radiation course, and chemotherapy regimen choice. Thus, a therapeutic option that could lower IL6 autocrine signaling with minimal toxic cellular effects would represent a potential chemo/radiation-sensitizer that could be used without affecting choices for adjuvant treatment.
Our research has shown that the compound 6,2′,4′-trimethoxyflavone (TMF) acts as an AHR antagonist without any partial agonist activity, unlike many other antagonists (26). For this reason, TMF could represent a therapeutically viable treatment for blocking AHR-mediated inflammatory effects without inducing DRE-mediated target genes, particularly those involved in Phase I xenobiotic metabolism. Although HNSCC encompasses a heterogeneous mixture of tumors, the ability of TMF treatment to reduce IL6 mRNA and secreted protein levels in multiple cell lines reinforces the hypothesis that high constitutive IL6 expression in these tumors is at least partly the result of a common AHR-mediated mechanism. Similarly, the variable level of endogenous AHR protein levels among three representative cell lines shows a loose correlation with basal IL6 and CYP1A1 transcription levels. Interestingly, nuclear levels of AHR protein have been tied to disease aggression and patient response rates in urothelial cancer (34). In this respect, the ability of 12 h, 10 μM TMF treatment to reduce both basal and induced levels of AHR-mediated IL6 demonstrates the viability of antagonist treatment, yet highlights the further need for compounds displaying stronger affinity in order to minimize dosing even in high AHR expressing lines. The lack of a more generalized anti-inflammatory response following TMF treatment supports a targeted mechanism of action at relevant doses.
The mechanism by which activated AHR synergistically induces IL6 in MCF-7 cells appears to be reflected in the HNSCC cell lines tested. Binding of the AHR to upstream DREs in the IL6 promoter has been shown to prime the DNA for NFκB-mediated induction through a dismissal of an HDAC-containing repressor complex. Our research shows that a basal level of AHR is bound to the IL6 promoter in HNSCC lines, thereby maintaining the DNA in a primed state that is readily accessible to transcriptional activators. In addition to the mechanism outlined previously, the identification of a role for the AHR in BRG1 occupancy and histone modification of the IL6 promoter simply adds to the complexity of the role that the AHR plays in IL6 transcriptional regulation.
On a molecular level, the AHR has been shown to have both ligand-dependent and independent effects on the transcription of cytoskeletal and migratory proteins including NEDD9, FASCIN-1, and VAV3, all of which are differentially transcribed in malignant cells (45–47). These effects highlight the extensive role that the receptor could play in cancerous tissue with respect to non-canonical target genes. Similarly, the AHR has also been implicated in a number of phenotypic end points across various cancer models. Agonist treatment has shown that activated AHR plays a role in increased breast cancer invasion (48), decreased contact inhibition in liver cells (49), and increased MMP9 expression and invasive ability in gastric cancer (50). Conversely, use of AHR antagonists or selective AHR modulators (SAhRMs) has been shown to inhibit prostate tumor metastases (51), breast cancer cell proliferation and tumor growth (52), and various inflammatory responses (53). A SAhRM has been identified that represses AHR-mediated inflammatory reactions without inducing CYP target gene activity. While some of these mechanisms remain undetermined, the expectation is that use of a SAhRM or antagonist would block numerous effects simply by competing for an activating ligand, thus reducing nuclear AHR levels. Elucidating the mechanisms by which a SAhRM or antagonist works, together with an understanding of the molecular interactions that lead to each AHR-dependent phenotypic endpoint would allow for rational treatment approaches to individual disease settings. For this reason, TMF treatment represents a potentially superior adjuvant therapy for HNSCC, as blocking constitutive pro-growth IL6 production may be only one of its beneficial endpoints. One simple outcome of AHR antagonist-receptor binding would be to block effects mediated by exogenous agonist exposure. The experiments performed in this study represent the level of constitutive AHR activation that is observed under controlled conditions in cell culture. In contrast, within an in vivo context, there are a diverse set of environmental sources of AHR ligands that humans are exposed to including air pollution, diet, and intestinal bacterial metabolites. For example, an important secondary effect of antagonist treatment would be to help reduce IL6 induction and other cellular effects initiated by B[a]P in HNSCC patients who smoke, or were previously exposed to B[a]P through manufacturing, car exhaust, or other sources. Patients who continue to smoke cigarettes while undergoing radiation therapy for head and neck cancer have been shown to have lower response rates and 2 year survival outcomes, as well as higher complications due to therapy (54, 55). Treatment of B[a]P-exposed patients with an AHR antagonist prior to radiation therapy, in conjunction with smoking cessation when applicable, could help raise outcome levels by blocking AHR-mediated anti-apoptotic effects that were the result of agonist exposure. Yet another source of AHR ligands is through the production of endogenous ligands during various disease states, and this concept is underscored by the recent discovery that indole metabolites (e.g. indoxyl sulfate, kynurenic acid) are potent human AHR ligands (56, 57).
The constitutively high cytokine expression found in HNSCC cell lines likely impacts the growth and anti-apoptotic effects common to these tumors when challenged with radiation and chemotherapy. Our findings that the AHR plays a key role in priming the IL6 promoter for expression, as well as the ability of antagonist treatment to significantly reduce cytokine expression in multiple lines, point to a potential role for the receptor in disease management. Use of an AHR antagonist has the potential to inhibit pro-growth and anti-apoptotic signaling, thus sensitizing the cells to more aggressive treatments typically employed. Further, AHR antagonist treatment would likely be free of the systemic side effects wrought by full organism blockades of cytokine signaling and major pathway inhibition. Modulation of AHR-induced IL6 and its autocrine loop is likely only one mechanism by which an antagonist or SAhRM would have an effect on tumor cell phenotype. Therefore, the role of an AHR antagonist on tumor aggressiveness and as a chemo/radio-sensitizer warrants further investigation.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant ES04869.
The authors would like to thank Dr. J. Silvio Gutkind for HNSCC cell lines, Dr. Susan Mallery for HNSCC cell lines as well as helpful discussions, and Dr. Steve Safe for TCDD.
References
- 1.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–50. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008 Jul 15;105(28):9721–6. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest. 2009 Jun;89(6):695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharat I, Saatcioglu F. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin are mediated by direct transcriptional interference with the liganded estrogen receptor. Cross-talk between aryl hydrocarbon- and estrogen-mediated signaling. J Biol Chem. 1996 May 3;271(18):10533–7. doi: 10.1074/jbc.271.18.10533. [DOI] [PubMed] [Google Scholar]
- 5.Furness SG, Whelan F. The pleiotropy of dioxin toxicity--xenobiotic misappropriation of the aryl hydrocarbon receptor’s alternative physiological roles. Pharmacol Ther. 2009 Dec;124(3):336–53. doi: 10.1016/j.pharmthera.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Safe S, Wormke M, Samudio I. Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells. J Mammary Gland Biol Neoplasia. 2000 Jul;5(3):295–306. doi: 10.1023/a:1009550912337. [DOI] [PubMed] [Google Scholar]
- 7.Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009 Feb 15;77(4):734–45. doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SH, Liang CT, Liu YW, Huang MC, Huang SC, Hong WF, Su JG. Crosstalk between activated forms of the aryl hydrocarbon receptor and glucocorticoid receptor. Toxicology. 2009 Aug 3;262(2):87–97. doi: 10.1016/j.tox.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, Hirokawa T, Akiyama T, Kurosumi M, Poellinger L, Kato S, Fujii-Kuriyama Y. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13481–6. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollingshead BD, Beischlag TV, DiNatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008 May 15;68(10):3609–17. doi: 10.1158/0008-5472.CAN-07-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiNatale BC, Schroeder JC, Francey LJ, Kusnadi A, Perdew GH. Mechanistic insights into the events that lead to synergistic induction of IL6 transcription upon activation of the Ah receptor and inflammatory signaling. J Biol Chem. 2010 Aug 6;285(32):24388–97. doi: 10.1074/jbc.M110.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavarretta IT, Neuwirt H, Untergasser G, Moser PL, Zaki MH, Steiner H, Rumpold H, Fuchs D, Hobisch A, Nemeth JA, Culig Z. The antiapoptotic effect of IL-6 autocrine loop in a cellular model of advanced prostate cancer is mediated by Mcl-1. Oncogene. 2007 May 3;26(20):2822–32. doi: 10.1038/sj.onc.1210097. [DOI] [PubMed] [Google Scholar]
- 13.Conze D, Weiss L, Regen PS, Bhushan A, Weaver D, Johnson P, Rincón M. Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res. 2001 Dec 15;61(24):8851–8. [PubMed] [Google Scholar]
- 14.Asgeirsson KS, Olafsdóttir K, Jónasson JG, Ogmundsdóttir HM. The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine. 1998 Sep;10(9):720–8. doi: 10.1006/cyto.1998.0349. [DOI] [PubMed] [Google Scholar]
- 15.Badache A, Hynes NE. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 2001 Jan 1;61(1):383–91. [PubMed] [Google Scholar]
- 16.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-{alpha}-positive human breast cancer. FASEB J. 2007 Nov;21(13):3763–70. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 17.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafe M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007 Dec;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, McCullagh L, Mousa S, Quezado M, Herscher LL, Van Waes C. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999 Jun;5(6):1369–79. [PubMed] [Google Scholar]
- 19.Pries R, Thiel A, Brocks C, Wollenberg B. Secretion of tumor-promoting and immune suppressive cytokines by cell lines of head and neck squamous cell carcinoma. In Vivo. 2006 Jan–Feb;20(1):45–8. [PubMed] [Google Scholar]
- 20.Woods KV, El-Naggar A, Clayman GL, Grimm EA. Variable expression of cytokines in human head and neck squamous cell carcinoma cell lines and consistent expression in surgical specimens. Cancer Res. 1998 Jul 15;58(14):3132–41. [PubMed] [Google Scholar]
- 21.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, Wolf GT, Teknos TN. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008 Aug 15;113(4):750–7. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 22.Tong M, Lloyd B, Pei P, Mallery SR. Human head and neck squamous cell carcinoma cells are both targets and effectors for the angiogenic cytokine, VEGF. J Cell Biochem. 2008 Dec 1;105(5):1202–10. doi: 10.1002/jcb.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinaldi AL, Morse MA, Fields HW, Rothas DA, Pei P, Rodrigo KA, Renner RJ, Mallery SR. Curcumin activates the aryl hydrocarbon receptor yet significantly inhibits (−)-benzo(a)pyrene-7R-trans-7,8-dihydrodiol bioactivation in oral squamous cell carcinoma cells and oral mucosa. Cancer Res. 2002 Oct 1;62(19):5451–6. [PubMed] [Google Scholar]
- 24.Yeudall WA, Crawford RY, Ensley JF, Robbins KC. MTS1/CDK4I is altered in cell lines derived from primary and metastatic oral squamous cell carcinoma. Carcinogenesis. 1994 Dec;15(12):2683–6. doi: 10.1093/carcin/15.12.2683. [DOI] [PubMed] [Google Scholar]
- 25.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9(1):R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray IA, Flaveny CA, DiNatale BC, Chairo CR, Schroeder JC, Kusnadi A, Perdew GH. Antagonism of aryl hydrocarbon receptor signaling by 6,2′,4′-trimethoxyflavone. J Pharmacol Exp Ther. 2010 Jan;332(1):135–44. doi: 10.1124/jpet.109.158261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Hankinson O. Functional involvement of the Brahma/SWI2-related gene 1 protein in cytochrome P4501A1 transcription mediated by the aryl hydrocarbon receptor complex. J Biol Chem. 2002 Apr 5;277(14):11821–7. doi: 10.1074/jbc.M110122200. [DOI] [PubMed] [Google Scholar]
- 28.Taylor RT, Wang F, Hsu EL, Hankinson O. Roles of coactivator proteins in dioxin induction of CYP1A1 and CYP1B1 in human breast cancer cells. Toxicol Sci. 2009 Jan;107(1):1–8. doi: 10.1093/toxsci/kfn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trotter KW, Archer TK. The BRG1 transcriptional coregulator. Nucl Recept Signal. 2008 Feb 1;6:e004. doi: 10.1621/nrs.06004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni Z, Karaskov E, Yu T, Callaghan SM, Der S, Park DS, Xu Z, Pattenden SG, Bremner R. Apical role for BRG1 in cytokine-induced promoter assembly. Proc Natl Acad Sci U S A. 2005 Oct 11;102(41):14611–6. doi: 10.1073/pnas.0503070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009 Jul;127(3):299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J Immunol. 2003 Mar 1;170(5):2374–81. doi: 10.4049/jimmunol.170.5.2374. [DOI] [PubMed] [Google Scholar]
- 33.Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010 Jan 27; doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida M, Mikami S, Kikuchi E, Kosaka T, Miyajima A, Nakagawa K, Mukai M, Okada Y, Oya M. Activation of the aryl hydrocarbon receptor pathway enhances cancer cell invasion by upregulating the MMP expression and is associated with poor prognosis in upper urinary tract urothelial cancer. Carcinogenesis. 2010 Feb;31(2):287–95. doi: 10.1093/carcin/bgp222. [DOI] [PubMed] [Google Scholar]
- 35.Chiaro CR, Morales JL, Prabhu KS, Perdew GH. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry. 2008 Aug 12;47(32):8445–55. doi: 10.1021/bi800712f. [DOI] [PubMed] [Google Scholar]
- 36.Chiaro CR, Patel RD, Perdew GH. 12(R)-Hydroxy-5(Z),8(Z),10(E),14(Z)-eicosatetraenoic acid [12(R)-HETE], an arachidonic acid derivative, is an activator of the aryl hydrocarbon receptor. Mol Pharmacol. 2008 Dec;74(6):1649–56. doi: 10.1124/mol.108.049379. [DOI] [PubMed] [Google Scholar]
- 37.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous Ah receptor ligand that synergistically induces interleukin 6 in the presence of inflammatory signaling. Toxicol Sci. 2010 May;115(1):89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder JC, DiNatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010 Jan 19;49(2):393–400. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003 Jun 1;63(11):2948–56. [PubMed] [Google Scholar]
- 40.Chakravarti N, Myers JN, Aggarwal BB. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane) Int J Cancer. 2006 Sep 15;119(6):1268–75. doi: 10.1002/ijc.21967. [DOI] [PubMed] [Google Scholar]
- 41.Leeman-Neill RJ, Wheeler SE, Singh SV, Thomas SM, Seethala RR, Neill DB, Panahandeh MC, Hahm ER, Joyce SC, Sen M, Cai Q, Freilino ML, Li C, Johnson DE, Grandis JR. Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis. 2009 Nov;30(11):1848–56. doi: 10.1093/carcin/bgp211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu F, Ma Y, Zhang Z, Zhao J, Kobayashi H, Zhang L, Fu L. Expression of Stat3 and Notch1 is associated with cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep. 2010 Mar;23(3):671–6. doi: 10.3892/or_00000683. [DOI] [PubMed] [Google Scholar]
- 43.Kondo N, Tsukuda M, Ishiguro Y, Kimura M, Fujita K, Sakakibara A, Takahashi H, Toth G, Matsuda H. Antitumor effects of lapatinib ( GW572016), a dual inhibitor of EGFR and HER-2, in combination with cisplatin or paclitaxel on head and neck squamous cell carcinoma. Oncol Rep. 2010 Apr;23(4):957–63. doi: 10.3892/or_00000720. [DOI] [PubMed] [Google Scholar]
- 44.Al-Sarraf M. Treatment of locally advanced head and neck cancer: historical and critical review. Cancer Control. 2002 Sep–Oct;9(5):387–99. doi: 10.1177/107327480200900504. [DOI] [PubMed] [Google Scholar]
- 45.Bui LC, Tomkiewicz C, Chevallier A, Pierre S, Bats AS, Mota S, Raingeaud J, Pierre J, Diry M, Transy C, Garlatti M, Barouki R, Coumoul X. Nedd9/Hef1/Cas-L mediates the effects of environmental pollutants on cell migration and plasticity. Oncogene. 2009 Oct 15;28(41):3642–51. doi: 10.1038/onc.2009.224. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto Y, Loftis DW, Adams JC. Fascin-1 promoter activity is regulated by CREB and the aryl hydrocarbon receptor in human carcinoma cells. PLoS One. 2009;4(4):e5130. doi: 10.1371/journal.pone.0005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carvajal-Gonzalez JM, Mulero-Navarro S, Roman AC, Sauzeau V, Merino JM, Bustelo XR, Fernandez-Salguero PM. The dioxin receptor regulates the constitutive expression of the vav3 proto-oncogene and modulates cell shape and adhesion. Mol Biol Cell. 2009 Mar;20(6):1715–27. doi: 10.1091/mbc.E08-05-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller ME, Holloway AC, Foster WG. Benzo-[a]-pyrene increases invasion in MDA-MB-231 breast cancer cells via increased COX-II expression and prostaglandin E2 (PGE2) output. Clin Exp Metastasis. 2005;22(2):149–56. doi: 10.1007/s10585-005-6536-x. [DOI] [PubMed] [Google Scholar]
- 49.Weiss C, Faust D, Schreck I, Ruff A, Farwerck T, Melenberg A, Schneider S, Oesch-Bartlomowicz B, Zatloukalová J, Vondrácek J, Oesch F, Dietrich C. TCDD deregulates contact inhibition in rat liver oval cells via Ah receptor, JunD and cyclin A. Oncogene. 2008 Apr 3;27(15):2198–207. doi: 10.1038/sj.onc.1210859. [DOI] [PubMed] [Google Scholar]
- 50.Peng TL, Chen J, Mao W, Song X, Chen MH. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009 Apr 16;10:27. doi: 10.1186/1471-2121-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fritz WA, Lin TM, Safe S, Moore RW, Peterson RE. The selective aryl hydrocarbon receptor modulator 6-methyl-1,3,8-trichlorodibenzofuran inhibits prostate tumor metastasis in TRAMP mice. Biochem Pharmacol. 2009 Apr 1;77(7):1151–60. doi: 10.1016/j.bcp.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, Lei P, Liu X, Li X, Walker K, Kotha L, Rowlands C, Safe S. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr Relat Cancer. 2009 Sep;16(3):835–44. doi: 10.1677/ERC-09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray IA, Krishnegowda G, DiNatale BC, Flaveny C, Chiaro C, Lin JM, Sharma AK, Amin S, Perdew GH. Development of a selective modulator of aryl hydrocarbon (Ah) receptor activity that exhibits anti-inflammatory properties. Chem Res Toxicol. 2010 May 17;23(5):955–66. doi: 10.1021/tx100045h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Browman GP, Wong G, Hodson I, Sathya J, Russell R, McAlpine L, Skingley P, Levine MN. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993 Jan 21;328(3):159–63. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 55.Zevallos JP, Mallen MJ, Lam CY, Karam-Hage M, Blalock J, Wetter DW, Garden AS, Sturgis EM, Cinciripini PM. Complications of radiotherapy in laryngopharyngeal cancer: effects of a prospective smoking cessation program. Cancer. 2009 Oct 1;115(19):4636–44. doi: 10.1002/cncr.24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schroeder JC, DiNatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010 Jan 19;49(2):393–400. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010 May;115(1):89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.