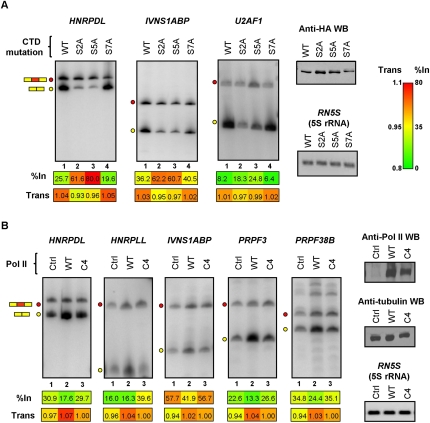
Figure 3.
Mutations of Pol II that affect transcription elongation consistently affect splicing and mRNA levels of RNA binding protein genes. (A) RT-PCR assays performed on total RNA isolated from Raji B cell lines expressing tetracycline-inducible, alpha-amanitin-resistant, and HA-tagged RNA Pol IIs harboring alanine-substituting mutations at every Ser 2, Ser 5, or Ser 7 position within the CTD heptapeptide repeats (see Results). The cell lines were treated with tetracycline to induce expression of the alpha-amanitin-resistant polymerases for 24 h, then endogenous Pol II was destroyed by treatment with alpha-amanitin for 48 h. Lysates from equal numbers of cells for each cell line were analyzed for Pol II levels by Western blotting using anti-HA antibody (Anti-HA WB) and total RNA was analyzed by RT-PCR assays as described in Figure 2. Numerical values below each panel represent average measurements from three independent experiments. Additional RT-PCR experiments and numerical quantification of the data are shown in Supplemental Figure S6. (B) Control (Ctrl) and expression plasmids for alpha-amanitin-resistant wild-type (WT) or C4 slow mutant (C4) polymerases were transiently expressed in 293T cells as described in Methods. RNA harvested from the transfected cells was analyzed by RT-PCR as described above. Expression levels of the exogenous Pol IIs were confirmed by Western blotting using an antibody (N-20), which recognizes the N-terminal domain of the largest subunit of Pol II. Tubulin detection was used as loading control for the Western blot, and 5S rRNA detection was used as loading control for RT-PCR assays. Numerical values below each panel represent average measurements from three independent experiments. Additional RT-PCR experiments and numerical quantification of the data are shown in Supplemental Figure S7.