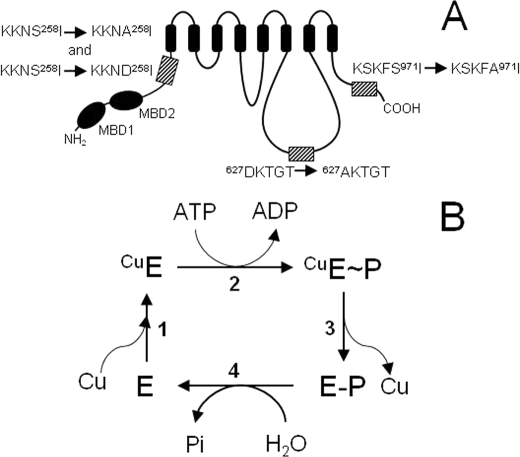
FIGURE 1.
Schematic representation of Ccc2 and its mutants, and proposed catalytic cycle of ATP hydrolysis and copper translocation. A, S258A, S258D, S971A, S258A/S971A, and D627A mutants were created by site-directed mutagenesis as described under “Experimental Procedures.” In S258A, S971A, and D627A, Ala replaces Ser258, Ser971, and Asp627, respectively, as highlighted by the hatched boxes in the representation of the Ccc2 topological structure. In S258D, Asp replaces Ser258. The small arrows between the sequences indicate the mutations and MDB1 and MDB2 represent the two metal-binding domains in the N-terminal region of Ccc2. For the sake of simplicity, the double-mutant S258A/S971A has been omitted. B, catalytic cycle proposed for Ccc2. Copper binding to the pump (step 1), phosphorylation by ATP (step 2), conversion of the high-energy E∼P form to the low-energy E-P form and copper release (step 3) and hydrolysis of the phosphorylated intermediate (step 4) are the main steps in the reaction that actively transports copper from the cytosol to acceptor proteins in the Golgi lumen (10).