FIGURE 7.
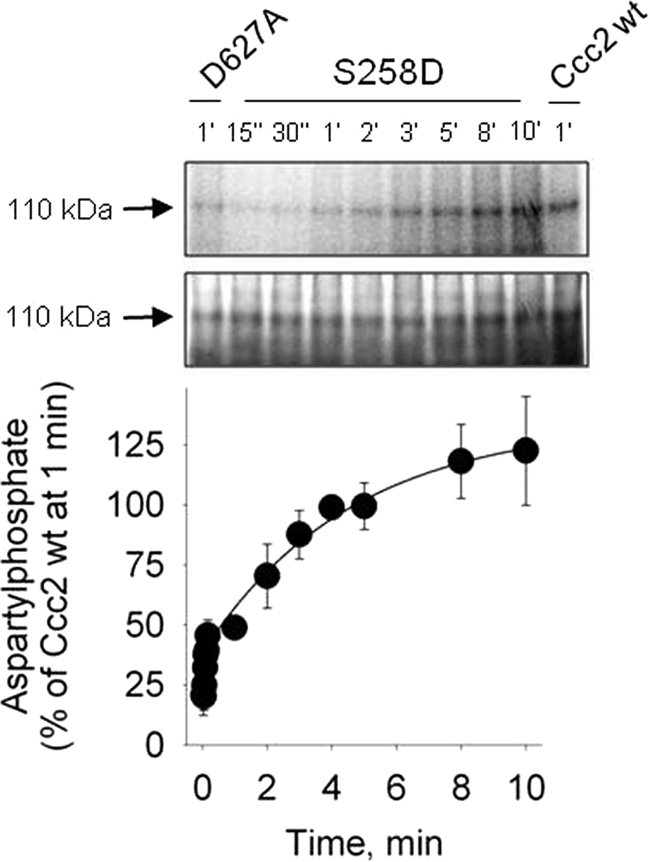
Time course of aspartylphosphate formation of the “phosphomimetic” mutant S258D. Top, autoradiogram of the 110-kDa band from a representative gel (at the indicated phosphorylation times) after resolution of the proteins by acidic gel electrophoresis. Membranes of Sf9 cells expressing S258D were phosphorylated with [γ-32P]ATP for the times indicated on the abscissa. Middle, Coomassie Blue staining of the same gels. Bottom, densitometric representation of the time course of phosphorylation obtained from autoradiograms corrected for the corresponding amount of protein loaded in each lane and for the background signal simultaneously measured with the non-phosphorylating D627A mutant. The phosphoenzyme levels were corrected for protein loading and background phosphosignal. For normalization, phosphorylation of Ccc2 wt at 1 min was taken as the reference (see “Experimental Procedures”). The smooth line was adjusted to the experimental points by fitting Equation 1 to the experimental values. Data are mean ± S.E. of four time course runs.
