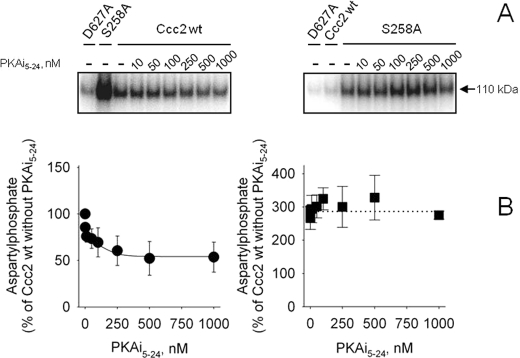
FIGURE 8.
PKA inhibition decreases aspartylphosphate formation at the catalytic cycle in Ccc2 wt but not in S258A. A, autoradiogram of the 110 kDa band from a representative gel after resolution of the proteins by acidic gel electrophoresis. Membranes of Sf9 cells expressing Ccc2 wt or S258A were phosphorylated for 1 min with [γ-32P]ATP and increasing concentrations of PKAi5–24, as indicated on the abscissa. B, densitometric representation of the autoradiograms demonstrating the levels of aspartylphosphate after 1 min in the presence of the PKAi5–24 concentrations given on the abscissa. Data are mean ± S.E. of >3 determinations. The data obtained from autoradiograms were corrected for the corresponding amount of protein loaded on each lane and for the background signal simultaneously measured with the non-phosphorylating D627A mutant (as shown on the top of the autoradiogram). For the inhibition of Ccc2 wt phosphorylation by PKAi5–24 the smooth line was adjusted to the experimental points, using the general equation of inhibition EPi = EPo × [I]/([I] + I0.5) + EPr, where EPi is the aspartylphosphate level at each PKAi5–24 concentration, EPo is the aspartylphosphate level in the absence of inhibitor, [I] is the concentration of PKAi5–24, I0.5 is the PKAi5–24 required for half-inhibition of the catalytic phosphorylation (50 nm) and EPr corresponds to the catalytic phosphorylation that is insensitive to PKA inhibition. The phosphoenzyme level of Ccc2 wt in the absence of PKA inhibitor was taken as the reference (100%).