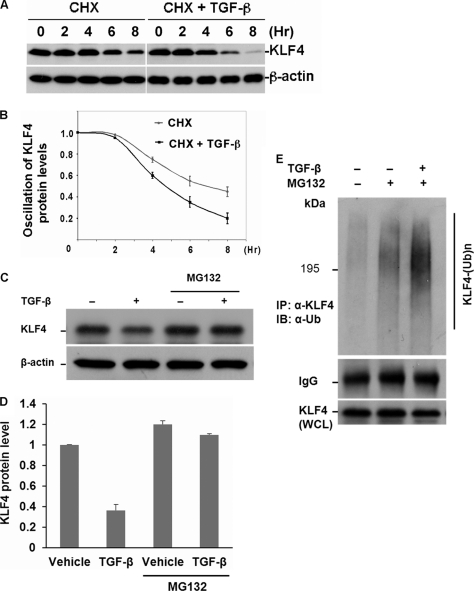
FIGURE 2.
TGF-β-induced KLF4 alteration is mediated by the ubiquitin-proteasome pathway. A, turnover rate of KLF4 protein in the absence and presence of TGF-β signaling. Mv1Lu cells were treated with 10 μg/ml of cycloheximide (CHX) only or CHX plus TGF-β. The cell pellet was collected at different time points as indicated. KLF4 protein levels were measured by immunoblotting. The KLF4 turnover rate was enhanced by stimulation with TGF-β. B, summary of A. C, TGF-β-induced KLF4 protein turnover is blocked by proteasomal inhibitor, MG132. Cells were collected 4 h after stimulation with TGF-β. The TGF-β-induced drop of KLF4 levels was attenuated by MG-132. D, quantification of C. E, TGF-β-induced KLF4 down-regulation is mediated by ubiquitylation. TGF-β-treated Mv1Lu cells were collected 4 h after cellular stimulation with TGF-β. The endogenous KLF4 protein complex was purified by immunoprecipitation using anti-KLF4 antibody coupled with protein A beads. The ubiquitin-conjugated KLF4 in the immunoprecipitation (IP) complex was detected by immunoblotting (IB) using anti-ubiquitin antibody. KLF4 protein levels in the whole cell lysates (WCL) were measured by immunoblotting. Obvious KLF4 ubiquitin conjugates were visualized in response to TGF-β signaling.